Inhibition of the NLRP3/IL-1β axis protects against sepsis-induced cardiomyopathy
- PMID: 34472725
- PMCID: PMC8718055
- DOI: 10.1002/jcsm.12763
Inhibition of the NLRP3/IL-1β axis protects against sepsis-induced cardiomyopathy
Abstract
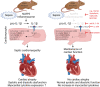
Background: Septic cardiomyopathy worsens the prognosis of critically ill patients. Clinical data suggest that interleukin-1β (IL-1β), activated by the NLRP3 inflammasome, compromises cardiac function. Whether or not deleting Nlrp3 would prevent cardiac atrophy and improve diastolic cardiac function in sepsis was unclear. Here, we investigated the role of NLRP3/IL-1β in sepsis-induced cardiomyopathy and cardiac atrophy.
Methods: Male Nlrp3 knockout (KO) and wild-type (WT) mice were exposed to polymicrobial sepsis by caecal ligation and puncture (CLP) surgery (KO, n = 27; WT, n = 33) to induce septic cardiomyopathy. Sham-treated mice served as controls (KO, n = 11; WT, n = 16). Heart weights and morphology, echocardiography and analyses of gene and protein expression were used to evaluate septic cardiomyopathy and cardiac atrophy. IL-1β effects on primary and immortalized cardiomyocytes were investigated by morphological and molecular analyses. IonOptix and real-time deformability cytometry (RT-DC) analysis were used to investigate functional and mechanical effects of IL-1β on cardiomyocytes.
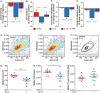
Results: Heart morphology and echocardiography revealed preserved systolic (stroke volume: WT sham vs. WT CLP: 33.1 ± 7.2 μL vs. 24.6 ± 8.7 μL, P < 0.05; KO sham vs. KO CLP: 28.3 ± 8.1 μL vs. 29.9 ± 9.9 μL, n.s.; P < 0.05 vs. WT CLP) and diastolic (peak E wave velocity: WT sham vs. WT CLP: 750 ± 132 vs. 522 ± 200 mm/s, P < 0.001; KO sham vs. KO CLP: 709 ± 152 vs. 639 ± 165 mm/s, n.s.; P < 0.05 vs. WT CLP) cardiac function and attenuated cardiac (heart weight-tibia length ratio: WT CLP vs. WT sham: -26.6%, P < 0.05; KO CLP vs. KO sham: -3.3%, n.s.; P < 0.05 vs. WT CLP) and cardiomyocyte atrophy in KO mice during sepsis. IonOptix measurements showed that IL-1β decreased contractility (cell shortening: IL-1β: -15.4 ± 2.3%, P < 0.001 vs. vehicle, IL-1RA: -6.1 ± 3.3%, P < 0.05 vs. IL-1β) and relaxation of adult rat ventricular cardiomyocytes (time-to-50% relengthening: IL-1β: 2071 ± 225 ms, P < 0.001 vs. vehicle, IL-1RA: 564 ± 247 ms, P < 0.001 vs. IL-1β), which was attenuated by an IL-1 receptor antagonist (IL-1RA). RT-DC analysis indicated that IL-1β reduced cardiomyocyte size (P < 0.001) and deformation (P < 0.05). RNA sequencing showed that genes involved in NF-κB signalling, autophagy and lysosomal protein degradation were enriched in hearts of septic WT but not in septic KO mice. Western blotting and qPCR disclosed that IL-1β activated NF-κB and its target genes, caused atrophy and decreased myosin protein in myocytes, which was accompanied by an increased autophagy gene expression. These effects were attenuated by IL-1RA.
Conclusions: IL-1β causes atrophy, impairs contractility and relaxation and decreases deformation of cardiomyocytes. Because NLRP3/IL-1β pathway inhibition attenuates cardiac atrophy and cardiomyopathy in sepsis, it could be useful to prevent septic cardiomyopathy.
Keywords: Heart failure; Interleukin-1 beta; NLR family, pyrin domain-containing 3 protein; Sepsis.
© 2021 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
K.B., M.K., N.H., T.E.K., M.S., A.Ha., S.G., M.T., S.S., B.C., M.W., St.G., D.B., A.He., C.S., C.B., S.B.F., F.C.L. and H.S. declare that they have no conflict of interest. The remaining authors have disclosed that they do not have any conflicts of interest.
Figures




References
-
- Vieillard‐Baron A, Cecconi M. Understanding cardiac failure in sepsis. Intensive Care Med 2014;40:1560–1563. - PubMed
-
- Charpentier J, Luyt CE, Fulla Y, Vinsonneau C, Cariou A, Grabar S, et al. Brain natriuretic peptide: a marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med 2004;32:660–665. - PubMed
-
- Vincent JL, Gris P, Coffernils M, Leon M, Pinsky M, Reuse C, et al. Myocardial depression characterizes the fatal course of septic shock. Surgery 1992;111:660–667. - PubMed
-
- Martin L, Derwall M, Al Zoubi S, Zechendorf E, Reuter DA, Thiemermann C, et al. The septic heart: current understanding of molecular mechanisms and clinical implications. Chest 2019;155:427–437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

