Evaluation of the SARS-CoV-2 Antibody Response to the BNT162b2 Vaccine in Patients Undergoing Hemodialysis
- PMID: 34473256
- PMCID: PMC8414193
- DOI: 10.1001/jamanetworkopen.2021.23622
Evaluation of the SARS-CoV-2 Antibody Response to the BNT162b2 Vaccine in Patients Undergoing Hemodialysis
Erratum in
-
Errors in Abstract and Figure 1.JAMA Netw Open. 2022 Feb 1;5(2):e221422. doi: 10.1001/jamanetworkopen.2022.1422. JAMA Netw Open. 2022. PMID: 35157065 Free PMC article. No abstract available.
Abstract
Importance: Patients undergoing hemodialysis have a high mortality rate associated with COVID-19, and this patient population often has a poor response to vaccinations. Randomized clinical trials for COVID-19 vaccines included few patients with kidney disease; therefore, vaccine immunogenicity is uncertain in this population.
Objective: To evaluate the SARS-CoV-2 antibody response in patients undergoing chronic hemodialysis following 1 vs 2 doses of BNT162b2 COVID-19 vaccination compared with health care workers serving as controls and convalescent serum.
Design, setting, and participants: A prospective, single-center cohort study was conducted between February 2 and April 17, 2021, in Toronto, Ontario, Canada. Participants included 142 patients receiving in-center hemodialysis and 35 health care worker controls.
Exposures: BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine.
Main outcomes and measures: SARS-CoV-2 IgG antibodies to the spike protein (anti-spike), receptor binding domain (anti-RBD), and nucleocapsid protein (anti-NP).
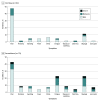
Results: Among the 142 participants undergoing maintenance hemodialysis, 94 (66%) were men; median age was 72 (interquartile range, 62-79) years. SARS-CoV-2 IgG antibodies were measured in 66 patients receiving 1 vaccine dose following a public health policy change, 76 patients receiving 2 vaccine doses, and 35 health care workers receiving 2 vaccine doses. Detectable anti-NP suggestive of natural SARS-CoV-2 infection was detected in 15 of 142 (11%) patients at baseline, and only 3 patients had prior COVID-19 confirmed by reverse transcriptase polymerase chain reaction testing. Two additional patients contracted COVID-19 after receiving 2 doses of vaccine. In 66 patients receiving a single BNT162b2 dose, seroconversion occurred in 53 (80%) for anti-spike and 36 (55%) for anti-RBD by 28 days postdose, but a robust response, defined by reaching the median levels of antibodies in convalescent serum from COVID-19 survivors, was noted in only 15 patients (23%) for anti-spike and 4 (6%) for anti-RBD in convalescent serum from COVID-19 survivors. In patients receiving 2 doses of BNT162b2 vaccine, seroconversion occurred in 69 of 72 (96%) for anti-spike and 63 of 72 (88%) for anti-RBD by 2 weeks following the second dose and median convalescent serum levels were reached in 52 of 72 patients (72%) for anti-spike and 43 of 72 (60%) for anti-RBD. In contrast, all 35 health care workers exceeded the median level of anti-spike and anti-RBD found in convalescent serum 2 to 4 weeks after the second dose.
Conclusions and relevance: This study suggests poor immunogenicity 28 days following a single dose of BNT162b2 vaccine in the hemodialysis population, supporting adherence to recommended vaccination schedules and avoiding delay of the second dose in these at-risk individuals.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

