SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19
- PMID: 34473343
- PMCID: PMC8411904
- DOI: 10.1002/14651858.CD013825.pub2
SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19
Abstract
Background: Monoclonal antibodies (mAbs) are laboratory-produced molecules derived from the B cells of an infected host. They are being investigated as a potential therapy for coronavirus disease 2019 (COVID-19).
Objectives: To assess the effectiveness and safety of SARS-CoV-2-neutralising mAbs for treating patients with COVID-19, compared to an active comparator, placebo, or no intervention. To maintain the currency of the evidence, we will use a living systematic review approach. A secondary objective is to track newly developed SARS-CoV-2-targeting mAbs from first tests in humans onwards. SEARCH METHODS: We searched MEDLINE, Embase, the Cochrane COVID-19 Study Register, and three other databases on 17 June 2021. We also checked references, searched citations, and contacted study authors to identify additional studies. Between submission and publication, we conducted a shortened randomised controlled trial (RCT)-only search on 30 July 2021.
Selection criteria: We included studies that evaluated SARS-CoV-2-neutralising mAbs, alone or combined, compared to an active comparator, placebo, or no intervention, to treat people with COVID-19. We excluded studies on prophylactic use of SARS-CoV-2-neutralising mAbs.
Data collection and analysis: Two authors independently assessed search results, extracted data, and assessed risk of bias using the Cochrane risk of bias tool (RoB2). Prioritised outcomes were all-cause mortality by days 30 and 60, clinical progression, quality of life, admission to hospital, adverse events (AEs), and serious adverse events (SAEs). We rated the certainty of evidence using GRADE.
Main results: We identified six RCTs that provided results from 17,495 participants with planned completion dates between July 2021 and December 2031. Target sample sizes varied from 1020 to 10,000 participants. Average age was 42 to 53 years across four studies of non-hospitalised participants, and 61 years in two studies of hospitalised participants. Non-hospitalised individuals with COVID-19 Four studies evaluated single agents bamlanivimab (N = 465), sotrovimab (N = 868), regdanvimab (N = 307), and combinations of bamlanivimab/etesevimab (N = 1035), and casirivimab/imdevimab (N = 799). We did not identify data for mortality at 60 days or quality of life. Our certainty of the evidence is low for all outcomes due to too few events (very serious imprecision). Bamlanivimab compared to placebo No deaths occurred in the study by day 29. There were nine people admitted to hospital by day 29 out of 156 in the placebo group compared with one out of 101 in the group treated with 0.7 g bamlanivimab (risk ratio (RR) 0.17, 95% confidence interval (CI) 0.02 to 1.33), 2 from 107 in the group treated with 2.8 g (RR 0.32, 95% CI 0.07 to 1.47) and 2 from 101 in the group treated with 7.0 g (RR 0.34, 95% CI 0.08 to 1.56). Treatment with 0.7 g, 2.8 g and 7.0 g bamlanivimab may have similar rates of AEs as placebo (RR 0.99, 95% CI 0.66 to 1.50; RR 0.90, 95% CI 0.59 to 1.38; RR 0.81, 95% CI 0.52 to 1.27). The effect on SAEs is uncertain. Clinical progression/improvement of symptoms or development of severe symptoms were not reported. Bamlanivimab/etesevimab compared to placebo There were 10 deaths in the placebo group and none in bamlanivimab/etesevimab group by day 30 (RR 0.05, 95% CI 0.00 to 0.81). Bamlanivimab/etesevimab may decrease hospital admission by day 29 (RR 0.30, 95% CI 0.16 to 0.59), may result in a slight increase in any grade AEs (RR 1.15, 95% CI 0.83 to 1.59) and may increase SAEs (RR 1.40, 95% CI 0.45 to 4.37). Clinical progression/improvement of symptoms or development of severe symptoms were not reported. Casirivimab/imdevimab compared to placebo Casirivimab/imdevimab may reduce hospital admissions or death (2.4 g: RR 0.43, 95% CI 0.08 to 2.19; 8.0 g: RR 0.21, 95% CI 0.02 to 1.79). We are uncertain of the effect on grades 3-4 AEs (2.4 g: RR 0.76, 95% CI 0.17 to 3.37; 8.0 g: RR 0.50, 95% CI 0.09 to 2.73) and SAEs (2.4 g: RR 0.68, 95% CI 0.19 to 2.37; 8.0 g: RR 0.34, 95% CI 0.07 to 1.65). Mortality by day 30 and clinical progression/improvement of symptoms or development of severe symptoms were not reported. Sotrovimab compared to placebo We are uncertain whether sotrovimab has an effect on mortality (RR 0.33, 95% CI 0.01 to 8.18) and invasive mechanical ventilation (IMV) requirement or death (RR 0.14, 95% CI 0.01 to 2.76). Treatment with sotrovimab may reduce the number of participants with oxygen requirement (RR 0.11, 95 % CI 0.02 to 0.45), hospital admission or death by day 30 (RR 0.14, 95% CI 0.04 to 0.48), grades 3-4 AEs (RR 0.26, 95% CI 0.12 to 0.60), SAEs (RR 0.27, 95% CI 0.12 to 0.63) and may have little or no effect on any grade AEs (RR 0.87, 95% CI 0.66 to 1.16). Regdanvimab compared to placebo Treatment with either dose (40 or 80 mg/kg) compared with placebo may decrease hospital admissions or death (RR 0.45, 95% CI 0.14 to 1.42; RR 0.56, 95% CI 0.19 to 1.60, 206 participants), but may increase grades 3-4 AEs (RR 2.62, 95% CI 0.52 to 13.12; RR 2.00, 95% CI 0.37 to 10.70). 80 mg/kg may reduce any grade AEs (RR 0.79, 95% CI 0.52 to 1.22) but 40 mg/kg may have little to no effect (RR 0.96, 95% CI 0.64 to 1.43). There were too few events to allow meaningful judgment for the outcomes mortality by 30 days, IMV requirement, and SAEs. Hospitalised individuals with COVID-19 Two studies evaluating bamlanivimab as a single agent (N = 314) and casirivimab/imdevimab as a combination therapy (N = 9785) were included. Bamlanivimab compared to placebo We are uncertain whether bamlanivimab has an effect on mortality by day 30 (RR 1.39, 95% CI 0.40 to 4.83) and SAEs by day 28 (RR 0.93, 95% CI 0.27 to 3.14). Bamlanivimab may have little to no effect on time to hospital discharge (HR 0.97, 95% CI 0.78 to 1.20) and mortality by day 90 (HR 1.09, 95% CI 0.49 to 2.43). The effect of bamlanivimab on the development of severe symptoms at day 5 (RR 1.17, 95% CI 0.75 to 1.85) is uncertain. Bamlanivimab may increase grades 3-4 AEs at day 28 (RR 1.27, 95% CI 0.81 to 1.98). We assessed the evidence as low certainty for all outcomes due to serious imprecision, and very low certainty for severe symptoms because of additional concerns about indirectness. Casirivimab/imdevimab with usual care compared to usual care alone Treatment with casirivimab/imdevimab compared to usual care probably has little or no effect on mortality by day 30 (RR 0.94, 95% CI 0.87 to 1.02), IMV requirement or death (RR 0.96, 95% CI 0.90 to 1.04), nor alive at hospital discharge by day 30 (RR 1.01, 95% CI 0.98 to 1.04). We assessed the evidence as moderate certainty due to study limitations (lack of blinding). AEs and SAEs were not reported. AUTHORS' CONCLUSIONS: The evidence for each comparison is based on single studies. None of these measured quality of life. Our certainty in the evidence for all non-hospitalised individuals is low, and for hospitalised individuals is very low to moderate. We consider the current evidence insufficient to draw meaningful conclusions regarding treatment with SARS-CoV-2-neutralising mAbs. Further studies and long-term data from the existing studies are needed to confirm or refute these initial findings, and to understand how the emergence of SARS-CoV-2 variants may impact the effectiveness of SARS-CoV-2-neutralising mAbs. Publication of the 36 ongoing studies may resolve uncertainties about the effectiveness and safety of SARS-CoV-2-neutralising mAbs for the treatment of COVID-19 and possible subgroup differences.
Trial registration: ClinicalTrials.gov NCT04501978 NCT04427501 NCT04545060 NCT04602000 NCT04381936 NCT04425629 NCT04505774 NCT04280705 NCT04401579 NCT04492475 NCT04602260 NCT04356495 NCT04561063 NCT04333732 NCT04534725 NCT04371367 NCT04382651 NCT04275245 NCT04341116 NCT04354428 NCT04369469 NCT04370262 NCT04415073 NCT04453384 NCT04454398 NCT04469179 NCT04494724 NCT04494984 NCT04497987 NCT04498273 NCT04516564 NCT04569786 NCT04574869 NCT04586153 NCT04629703 NCT04766671 NCT04859517 NCT04894474 NCT04333420 NCT04621149 NCT04625972 NCT04429529 NCT04441918 NCT04441931 NCT04479631 NCT04479644 NCT04483375 NCT04507256 NCT04519437 NCT04525079 NCT04532294 NCT04533048 NCT04537910 NCT04561076 NCT04567810 NCT04590430 NCT04592549 NCT04603651 NCT04617535 NCT04656691 NCT04691180 NCT04700163 NCT04701658 NCT04896541 NCT04932850 NCT04351724 NCT04593940 NCT04640168 NCT04583956 NCT04583969 NCT04355143 NCT04662086 NCT04324047 NCT04328012 NCT04473053 NCT04891133 NCT04488081 NCT04590586 NCT04452318 NCT04703608 NCT04386070 NCT02735707 NCT04826588 NCT04393246 NCT04390464 NCT04445467 NCT04518410 NCT04746183 NCT04315948 NCT04411628 NCT04426695 NCT04551898 NCT04584697 NCT04593641 NCT04627584 NCT04631666 NCT04631705 NCT04634409 NCT04644120 NCT04644185 NCT04649515 NCT04666441 NCT04674566 NCT04683328 NCT04709328 NCT04723394 NCT04734860 NCT04748588 NCT04770467 NCT04771351 NCT04779879 NCT04780321 NCT04787211 NCT04796402 NCT04805671 NCT04822701 NCT04900428 NCT04913675 NCT04952805 NCT04790786.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
NK: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'COVIM', which was paid to the institution).
CH: none known
KLC: none known
ET: none known
ZK: none known
MP: none known
MN: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'COVIM', which was paid to the institution).
VP: none known
SS: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'COVIM' which was paid to the institution).
SJV: receives a PhD scholarship from the not‐for‐profit Sanquin blood bank.
IM: none known
CS: none known
EMW: none known
CS‐O: has declared to be employed by the not‐for‐profit Sanquin blood bank.
DJR: is a consultant haematologist for NHS Blood and Transplant.
ZM: is a haematologist at Monash University.
LJE: is a consultant haematologist for NHS Blood and Transplant.
NS: none known
Figures


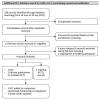




















































Update of
References
References to studies included in this review
ACTIV‐3 {published data only}
BLAZE‐1 (phase 2, 0.7g) {published data only}
-
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325(7):632-44. [DOI: 10.1001/jama.2021.0202] - DOI - PMC - PubMed
BLAZE‐1 (phase 2, 2.8g) {published data only}
-
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325(7):632-44. [DOI: 10.1001/jama.2021.0202] - DOI - PMC - PubMed
BLAZE‐1 (phase 2, 7.0g) {published data only}
-
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325(7):632-44. [DOI: 10.1001/jama.2021.0202] - DOI - PMC - PubMed
BLAZE‐1 (phase 2) {published data only}
-
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325(7):632-44. [DOI: 10.1001/jama.2021.0202] - DOI - PMC - PubMed
BLAZE‐1 (phase 3) {published data only}
-
- Dougan M, Nirula A, Gottlieb RL, Azizad M, Mocherla B, Chen P, et al. Bamlanivimab + etesevimab for treatment of COVID-19 in high-risk ambulatory patients. Topics in Antiviral Medicine 2021;29(1):33, abstract no. 122.
COMET‐ICE {published data only}
Eom 2021 {published data only}
-
- Eom JS, Ison M, Streinu-Cercel A, Săndulescu O, Preotescu LL, Kim YS, et al. Efficacy and safety of CT-P59 plus standard of care: a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate SARS-CoV-2 infection. researchsquare.com/article/rs-296518/v1. [DOI: 10.21203/rs.3.rs-296518/v1] - DOI
Eom 2021 (40 mg/kg) {published data only}
-
- Eom JS, Ison M, Streinu-Cercel A, Săndulescu O, Preotescu LL, Kim YS, et al. Efficacy and safety of CT-P59 plus standard of care: a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate SARS-CoV-2 infection. researchsquare.com/article/rs-296518/v1. [DOI: 10.21203/rs.3.rs-296518/v1] - DOI
Eom 2021 (80 mg/kg) {published data only}
-
- Eom JS, Ison M, Streinu-Cercel A, Săndulescu O, Preotescu LL, Kim YS, et al. Efficacy and safety of CT-P59 plus standard of care: a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate SARS-CoV-2 infection. researchsquare.com/article/rs-296518/v1. [DOI: 10.21203/rs.3.rs-296518/v1] - DOI
RECOVERY {published data only}
-
- Horby PW, Mafham M, Peto L, Campbell M, Pessoa-Amorim G, Spata E, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.06.15.21258542] - DOI
Weinreich (phase 1/2, 2.4 g) {published data only}
-
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGEN-COV antibody cocktail in outpatients with COVID-19. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.06.09.21257915] - DOI
Weinreich (phase 1/2, 8.0 g) {published data only}
-
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGEN-COV antibody cocktail in outpatients with COVID-19. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.06.09.21257915] - DOI
Weinreich (phase 1/2) {published data only}
-
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGEN-COV antibody cocktail in outpatients with COVID-19. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.06.09.21257915] - DOI
Weinreich (phase 3) {published data only}
-
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGEN-COV antibody cocktail clinical outcomes study in COVID-19 outpatients. medRxiv [Preprint]. [DOI: 10.1101/2021.05.19.21257469] - DOI
References to studies excluded from this review
ACTIV‐4 {published data only}
-
- NCT04505774. Anti-thrombotics for adults hospitalized with COVID-19 (ACTIV-4). clinicaltrials.gov/ct2/show/NCT04505774 (first received 10 August 2020).
ACTT {published data only}
-
- Euctr2020-001052-18-de. A multicenter, adaptive, randomised blinded controlled trial of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalized adults - version for European Union/United Kingdom sites. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001052-18/DK (first received 20 March 2020).
-
- ISRCTN13035264. Adaptive COVID-19 treatment trial in the EU & UK. www.isrctn.com/ISRCTN13035264 (first received 11 July 2020).
-
- NCT04280705. Adaptive COVID-19 treatment trial. clinicaltrials.gov/ct2/show/NCT04280705 (first received 25 September 2020).
ACTT‐2 {published data only}
-
- NCT04401579. Adaptive COVID-19 treatment trial 2 (ACTT-II). clinicaltrials.gov/ct2/show/NCT04401579 (first received 26 May 2020).
ACTT‐3 {published data only}
-
- NCT04492475. Adaptive COVID-19 treatment trial 3 (ACTT-3). clinicaltrials.gov/ct2/show/NCT04492475 (first received 30 July 2020).
Alam 2021 {published data only}
-
- Alam MM, Mahmud S, Aggarwal S, Fathma S, Al Mahi N, Shibli MS, et al. Clinical impact of the early use of monoclonal antibody LY-CoV555 (bamlanivimab) on mortality and hospitalization among elderly nursing home patients: a multicenter retrospective study. Cureus 2021;13(5):e14933. [DOI: 10.7759/cureus.14933] - DOI - PMC - PubMed
AMMURAVID {published data only}EUCTR2020‐001854‐23
-
- EUCTR2020-001854-23. A study with immunotherapy for moderate COVID-19. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001854-23/IT (first received 26 June 2020).
ASCOT‐ADAPT {published data only}
-
- ACTRN12620000445976. Australasian COVID-19 trial: an adaptive platform trial (ASCOT-ADAPT). A multi-centre randomised adaptive platform clinical trial to assess clinical, virological and immunological outcomes in patients with SARS-CoV-2 infection (COVID-19). anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620000445976 (first received 2 April 2020).
-
- NCT04483960. Australasian COVID-19 trial (ASCOT) adaptive platform trial. clinicaltrials.gov/ct2/show/NCT04483960 (first received 23 July 2020).
Bariola 2021 {published data only}
-
- Bariola JR, McCreary EK, Wadas RJ, Kip KE, Marroquin OC, Minnier T, et al. Impact of monoclonal antibody treatment on hospitalization and mortality among non-hospitalized adults with SARS-CoV-2 infection. Open Forum Infectious Diseases 2021;8(7):ofab254. [DOI: 10.1093/ofid/ofab254] - DOI - PMC - PubMed
Beam 2021 {published data only}
BLAZE‐2 {published data only}
-
- NCT04497987. A study of LY3819253 (LY-CoV555) and LY3832479 (LY-CoV016) in preventing SARS-CoV-2 infection and COVID-19 in nursing home residents and staff (BLAZE-2). clinicaltrials.gov/ct2/show/NCT04497987 (first received 4 August 2020).
ChiCTR2000030012 {published data only}
-
- ChiCTR2000030012. Development of anti-2019-nCoV therapeutic antibody from the recovered novel coronavirus pneumonia patients (COVID-19). www.chictr.org.cn/showproj.aspx?proj=49718 (first received 19 February 2020).
Cohen 2021 {published data only}
COREG {published data only}
-
- NCT04602260. Functional recovery of hospitalised patients with COVID-19: the COREG extension study. clinicaltrials.gov/show/NCT04602260 (first received 26 October 2020).
COVERAGE {published data only}
-
- Duvignaud A, Lhomme E, Pistone T, Onaisi R, Sitta R, Journot V, et al. Home treatment of older people with symptomatic SARS-CoV-2 infection (COVID-19): a structured summary of a study protocol for a multi-arm multi-stage (MAMS) randomized trial to evaluate the efficacy and tolerability of several experimental treatments to reduce the risk of hospitalisation or death in outpatients aged 65 years or older (COVERAGE trial). Trials 2020;21(1):846. - PMC - PubMed
-
- NCT04356495. Treatments to decrease the risk of hospitalization or death in elderly outpatients with symptomatic SARS-CoV-2 infection (COVID-19). clinicaltrials.gov/ct2/show/NCT04356495 (first received 22 April 2020).
COVER HCW {published data only}
-
- NCT04561063. COVID-19 prophylaxis South Africa (COVER HCW). clinicaltrials.gov/ct2/show/NCT04561063 (first received 23 September 2020).
COVID_Aging {published data only}EUCTR2020‐001303‐16‐FR
-
- EUCTR2020-001303-16-FR. Efficacy of hydroxychloroquine, telmisartan and azithromycin on survival in elderly hospitalized patients with VIDOC-19: a randomized, multi-centre, adaptive, blinded study. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001303-16/FR (first received 29 March 2020).
CROWN CORONA {published data only}
-
- NCT04333732. Crown coronation: COVID-19 research outcomes worldwide network for CORONAvirus prevention (CROWN CORONA). clinicaltrials.gov/ct2/show/NCT04333732 (first received 3 April 2020).
C‐SMART {published data only}
-
- NCT04534725. COVID-19 prevention and treatment in cancer; a sequential multiple assignment randomised trial. clinicaltrials.gov/show/NCT04534725 (first received 1 September 2020).
Dale 2021 {published data only}
-
- Dale AP, Hudson M, Cullen T, Ellingson K, Davis K, Armenta D, et al. Administration of bamlanivimab to skilled nursing facility residents during a COVID-19 outbreak, January-February 2021, Arizona. Journal of the American Medical Directors Association 2021;S1525-8610(21):00422-9. [DOI: 10.1016/j.jamda.2021.04.023] - DOI - PMC - PubMed
Dhand 2021a {published data only}
Dhand 2021b {published data only}
Dong 2021 {published data only}
-
- Dong J, Zost SJ, Greaney AJ, Starr TN, Dingens AS, Chen EC, et al. Genetic and structural basis for recognition of SARS-CoV-2 spike protein by a two-antibody cocktail. bioRxiv [Preprint] 2021. [DOI: 10.1101/2021.01.27.428529] - DOI
EUCTR2020‐001243‐15‐BE {published data only}EUCTR2020‐001243‐15‐BE
-
- EUCTR2020-001243-15-BE. COVID-19: a randomized, open-label, adaptive, proof-of-concept clinical trial of new antiviral drug candidates against SARS-CoV-2. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001243-15/BE (first received 27 March 2020).
EUDRACT2020‐002713‐17 {published data only}EUDRACT2020‐002713‐17
-
- EUDRACT2020-002713-17. A study to evaluate the safety and efficacy of MSTT1041A or UTTR1147A in patients with severe COVID-19 pneumonia. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:202... (first received 13 July 2020).
FORCE {published data only}EUDRACT2020‐001686‐36
-
- NCT04371367. Avdoralimab an anti-C5aR antibody, in patients with COVID-19 severe pneumonia. clinicaltrials.gov/show/NCT04371367 (first received 1 May 2020).
Ganesh 2021 {published data only}
-
- Ganesh R, Pawlowski C, O'Horo JC, Arndt LL, Arndt R, Bell SJ, et al. Association of intravenous bamlanivimab use with reduced hospitalization, intensive care unit admission, and mortality in patients with mild to moderate COVID-19. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.05.23.21257670] - DOI - PMC - PubMed
jRCT2031190264 {published data only}jRCT2031190264
-
- jRCT2031190264. A multicenter, adaptive, randomized blinded controlled trial of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalized adults. jrct.niph.go.jp/latest-detail/jRCT2031190264 2020.
Karr 2021 {published data only}
Kutzler 2021 {published data only}
MAS‐COVID {published data only}
-
- NCT04382651. Study of efficacy and safety of MAS825 in patients with COVID-19 (MAS-COVID). clinicaltrials.gov/ct2/show/NCT04382651 (first received 11 May 2020).
NCT04275245 {published data only}
-
- NCT04275245. Clinical study of anti-CD147 humanized meplazumab for injection to treat with 2019-nCoV pneumonia. ClinicalTrials.gov/show/NCT04275245 (first received 19 February 2020).
NCT04341116 {published data only}
-
- NCT04341116. Study of TJ003234 (anti-GM-CSF monoclonal antibody) in subjects with severe coronavirus disease 2019 (COVID-19). clinicaltrials.gov/show/NCT04341116 (first received 10 April 2020).
NCT04354428 {published data only}
-
- NCT04354428. Treatment for SARS-CoV-2 in high-risk adult outpatients. clinicaltrials.gov/ct2/show/NCT04354428 (first received 21 April 2020).
NCT04369469 {published data only}
-
- NCT04369469. Efficacy and safety study of IV ravulizumab in patients with COVID-19 severe pneumonia. clinicaltrials.gov/show/NCT04369469 (first received 30 April 2020).
NCT04370262 {published data only}
-
- NCT04370262. Multi-site adaptive trials for COVID-19. clinicaltrials.gov/ct2/show/NCT04370262 (first received 30 April 2020).
NCT04415073 {published data only}
-
- NCT04415073. A phase 2 study to evaluate axatilimab for hospitalized patients with respiratory involvement secondary to COVID-19. clinicaltrials.gov/show/NCT04415073 (first received 4 June 2020).
NCT04452318 {published data only}
-
- NCT04452318. Study assessing the efficacy and safety of anti-spike SARS CoV-2 monoclonal antibodies for prevention of SARS CoV-2 infection asymptomatic in healthy adults who are household contacts to an individual with a positive SARS-CoV-2 RT-PCR assay. clinicaltrials.gov/ct2/show/NCT04452318 (first received 30 June 2020).
NCT04453384 {published data only}
-
- NCT04453384. Study to evaluate the safety and efficacy of XAV-19 in patients with COVID-19 induced moderate pneumonia. clinicaltrials.gov/show/NCT04453384 (first received 1 July 2020).
NCT04454398 {published data only}
-
- NCT04454398. A randomized placebo-controlled study to evaluate STI-1499 (COVI-GUARD) in hospitalized patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04454398 (first received 1 July 2020).
NCT04469179 {published data only}
-
- NCT04469179. Safety, tolerability, and pharmacokinetics of SAB-185 in ambulatory participants with COVID-19. clinicaltrials.gov/ct2/show/NCT04469179 (first received 13 July 2020).
NCT04494724 {published data only}
-
- NCT04494724. Clazakizumab vs. placebo - COVID-19 infection. clinicaltrials.gov/show/NCT04494724 (first received 31 July 2020).
NCT04494984 {published data only}
-
- NCT04494984. A study to investigate the pharmacokinetics, efficacy and safety of INM005 in patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04494984 (first received 31 July 2020).
NCT04497987 {published data only}
-
- A study of LY3819253 (LY-CoV555) and LY3832479 (LY-CoV016) in preventing SARS-CoV-2 infection and COVID-19 in nursing home residents and staff. clinicaltrials.gov/show/NCT04497987.
NCT04498273 {published data only}
-
- NCT04498273. COVID-19 positive outpatient thrombosis prevention in adults aged 40-80. clinicaltrials.gov/ct2/show/NCT04498273 (first received 4 August 2020).
NCT04514302 {published data only}
-
- NCT04514302. Safety and efficacy of anti-SARS-CoV-2 equine antibody fragments (INOSARS) for hospitalized patients with COVID-19. clinicaltrials.gov/show/NCT04514302 (first received 14 August 2020).
NCT04516564 {published data only}
-
- A study of AK119 (anti-CD73 antibody), a treatment for COVID-19, in healthy subjects. clinicaltrials.gov/show/NCT04516564 (first received 18 August 2020).
NCT04569786 {published data only}
-
- Dose ranging trial to assess safety and immunogenicity of V590 (COVID-19 vaccine) in healthy adults (V590-001). clinicaltrials.gov/show/NCT04569786 (first received 30 September 2020).
NCT04574869 {published data only}
-
- NCT04574869. A study of RLS-0071 in patients with acute lung injury due to COVID-19 pneumonia in early respiratory failure. clinicaltrials.gov/ct2/show/NCT04574869 (first received 5 October 2020).
NCT04586153 {published data only}
-
- NCT04586153. Study to assess the effect of meplazumab on COVID-19. clinicaltrials.gov/show/NCT04586153 (first received 14 October 2020).
NCT04625725 {published data only}
-
- NCT04625725. Phase III double-blind, placebo-controlled study of AZD7442 for pre-exposure prophylaxis of COVID-19 in adult. clinicaltrials.gov/ct2/show/NCT04625725 (first received 12 November 2020).
NCT04629703 {published data only}
-
- NCT04629703. Double-blind, randomized, placebo-controlled, adaptive design, multi-center phase 3 study to evaluate the efficacy and safety of fostamatinib in COVID-19 subjects. clinicaltrials.gov/ct2/show/NCT04629703 (first received 16 November 2020).
NCT04766671 {published data only}
-
- NCT04766671. An exploratory study to describe virological effect, safety, and pharmacokinetics of VIR-7831 monoclonal antibody in hospitalized participants with COVID-19. inclinicaltrials.com/covid-19/NCT04766671/.
NCT04859517 {published data only}
-
- NCT04859517. Evaluation of ADG20 for the prevention of COVID-19. clinicaltrials.gov/show/NCT04859517.
NCT04894474 {published data only}
-
- NCT04894474. A study to test whether BI 767551 can prevent COVID-19 in people who have been exposed to SARS-CoV-2. clinicaltrials.gov/show/NCT04894474.
PANAMO {published data only}
-
- NCT04333420. Randomized, controlled study of IFX-1 in patients with severe COVID-19 pneumonia (PANAMO). clinicaltrials.gov/ct2/show/NCT04333420 (first received 3 April 2020).
PO‐COV‐III‐20 {published data only}EUCTR2020‐001782‐37‐SK
-
- EUCTR2020-001782-37-SK. A multi-centre, adaptive, randomized, double-blind, placebo-controlled comparative clinical study of the safety and efficacy of 12 mg Polyoxidonium®, lyophilizate solution for injections (NPO Petrovax Pharm LLC, Russia) in patients with coronavirus disease (COVID-19). www.clinicaltrialsregister.eu/ctr-search/trial/2020-001782-37/SK (first received 15 June 2020).
PROFACT‐01 {published data only}
-
- NCT04621149. An outpatient study investigating non-prescription treatments for COVID-19 (PROFACT-01). clinicaltrials.gov/ct2/show/NCT04621149 (first received 9 November 2020).
Rainwater‐Lovett 2021 {published data only}
RESP301‐002 {published data only}EUCTR2020‐002120‐37‐GB
-
- EUCTR2020-002120-37-GB. An open-label, adaptive randomized, controlled multicenter study to evaluate the efficacy and safety of RESP301+SOC vs SOC in hospitalized participants with COVID-19 requiring supplemental oxygen. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001072-15/GB (first received 19 March 2020).
Shirk 2021 {published data only}
STORM CHASER {published data only}
-
- NCT04625972. Phase III double-blind, placebo-controlled study of AZD7442 for post- exposure prophylaxis of COVID-19 in adults (STORM CHASER). clinicaltrials.gov/show/NCT04625972 (first received 12 November 2020).
Track: ChiCTR2100042150 {published data only}
-
- ChiCTR2100042150. Phase I clinical study to evaluate the safety, tolerability, pharmacokinetic profile and immunogenicity of JMB-2002 injection in Chinese healthy subjects after intravenous infusion of single dose. www.chictr.org.cn/showprojen.aspx?proj=66096.
Track: jRCT2071200117 {published data only}
-
- jRCT2071200117. A phase 1 study of casavirimab and imdevimab in Japanese adult volunteers. rctportal.niph.go.jp/en/detail?trial_id=jRCT2071200117.
Track: NCT04429529 {published data only}
-
- NCT04429529. Safety of TY027, a treatment for COVID-19, in humans. clinicaltrials.gov/ct2/show/NCT04429529 (first received 12 June 2020).
Track: NCT04441918 {published data only}
-
- NCT04441918. Tolerability, safety, pharmacokinetic profile and immunogenicity of a recombinant humanized anti-SARS-CoV-2 monoclonal antibody (JS016) for injection in Chinese health subjects. clinicaltrials.gov/ct2/show/NCT04441918 (first received 22 June 2020).
Track: NCT04441931 {published data only}
-
- NCT04441931. A study of LY3832479 (LY-CoV016) in healthy participants. clinicaltrials.gov/ct2/show/NCT04441931 (first received 22 June 2020).
Track: NCT04479631 {published data only}
-
- NCT04479631. Safety, tolerability, and pharmacokinetics study of human monoclonal antibody BRII-196. clinicaltrials.gov/ct2/show/NCT04479631 (first received 21 July 2020).
Track: NCT04479644 {published data only}
-
- NCT04479644. Safety, tolerability, and pharmacokinetics study of human monoclonal antibody BRII-198. clinicaltrials.gov/ct2/show/NCT04479644 (first received 21 July 2020).
Track: NCT04483375 {published data only}
-
- NCT04483375. Safety, tolerability and pharmacokinetics of SCTA01, an anti-COVID-19 monoclonal antibody, in healthy Chinese subjects. clinicaltrials.gov/ct2/show/NCT04483375 (first received July 2020).
Track: NCT04507256 {published data only}
-
- NCT04507256. Study to evaluate the safety, tolerability and pharmacokinetics of AZD7442 in healthy adults. clinicaltrials.gov/ct2/show/NCT04507256 (first received 11 August 2020).
Track: NCT04519437 {published data only}
-
- NCT04519437. Study assessing the safety, tolerability, pharmacokinetics, and immunogenicity of repeated subcutaneous doses of anti-spike (S) SARS-CoV-2 monoclonal antibodies (REGN10933+REGN10987) in adult volunteers as related to COVID-19. clinicaltrials.gov/ct2/show/NCT04519437 (first received 19 August 2020).
Track: NCT04525079 {published data only}
-
- NCT04525079. To evaluate the safety, tolerability and pharmacokinetics of CT-P59 in healthy subjects. clinicaltrials.gov/ct2/show/NCT04525079 (first received 25 August 2020).
Track: NCT04532294 {published data only}
-
- NCT04532294. Safety, tolerability, pharmacokinetics, and immunogenicity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibody in healthy subjects. clinicaltrials.gov/ct2/show/NCT04532294 (first received 31 August 2020).
Track: NCT04533048 {published data only}
-
- NCT04533048. A clinical study to evaluate MW33 injection. clinicaltrials.gov/ct2/show/NCT04533048 (first received 31 August 2020).
Track: NCT04537910 {published data only}
-
- NCT04537910. A study of LY3819253 (LY-CoV555) in healthy participants. clinicaltrials.gov/ct2/show/NCT04537910 (first received 3 September 2020).
Track: NCT04561076 {published data only}
-
- NCT04561076. Evaluate safety and pharmacokinetics of HLX70 in healthy adult volunteers. clinicaltrials.gov/ct2/show/NCT04561076 (first received 23 September 2020).
Track: NCT04567810 {published data only}
-
- NCT04567810. Safety, tolerability, and pharmacokinetics of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) chicken egg antibody (IgY) (COVID-19). clinicaltrials.gov/ct2/show/NCT04567810 (first received 29 September 2020).
Track: NCT04590430 {published data only}
-
- NCT04590430. Study to assess the safety, tolerability, and pharmacokinetics of HFB30132A against COVID-19 in healthy adults. clinicaltrials.gov/ct2/show/NCT04590430 (first received 19 October 2020).
Track: NCT04592549 {published data only}
-
- NCT04592549. Study of monoclonal antibody cocktail being tested for the prevention of COVID-19 [A phase 1, randomized, double-blind, placebo-controlled, dose escalation study to evaluate the safety, pharmacokinetics, and immunogenicity of ADM03820 in adults]. clinicaltrials.gov/ct2/show/NCT04592549 (first received 19 October 2020).
Track: NCT04603651 {published data only}
-
- NCT04603651. Expanded access program to provide bamlanivimab (LY3819253) for the treatment of COVID-19. clinicaltrials.gov/ct2/show/NCT04603651 (first received 27 October 2020).
Track: NCT04617535 {published data only}
-
- NCT04617535. Compassionate use of REGN-COV2 for the treatment of COVID-19. clinicaltrials.gov/ct2/show/NCT04617535 (first received 5 November 2020).
Track: NCT04656691 {published data only}
-
- NCT04656691. At-home infusion using bamlanivimab in participants with mild to moderate COVID-19 (UNITED). clinicaltrials.gov/ct2/show/NCT04656691.
Track: NCT04691180 {published data only}
-
- NCT04691180. A phase 1 study of human monoclonal antibodies, BRII-196 and BRII-198. clinicaltrials.gov/ct2/show/NCT04691180 (first received 31 December 2020). [CLINICALTRIALS.GOV: NCT04691180]
Track: NCT04700163 {published data only}
-
- NCT04700163. RU anti-SARS-CoV-2 mAbs in healthy volunteers. clinicaltrials.gov/ct2/show/NCT04700163 (first received 7 January 2021).
Track: NCT04701658 {published data only}
-
- NCT04701658. A real world study of bamlanivimab in participants with mild-to-moderate coronavirus disease 2019 (COVID-19). clinicaltrials.gov/ct2/show/NCT04701658 (first received 8 January 2021).
Track: NCT04852978 {published data only}
-
- NCT04852978. COVID-19 study to assess immunogenicity, safety, and tolerability of Moderna mRNA-1273 vaccine administered with casirivimab+imdevimab in healthy adult volunteers. clinicaltrials.gov/ct2/show/NCT04852978.
Track: NCT04896541 {published data only}
-
- NCT04896541. Phase I double-blind, placebo-controlled study of AZD7442. https://clinicaltrials.gov/ct2/show/NCT04896541.
Track: NCT04932850 {published data only}
-
- NCT04932850. Study to evaluate the safety and concentrations of monoclonal antibody against virus that causes COVID-19 disease. clinicaltrials.gov/ct2/show/NCT04932850.
Webb 2021 {published data only}
Yang 2020 {published data only}
-
- Yang X, Pan Y, Song G, Wang J, Zhan S, Wu X, et al. Anti-SARS-CoV-2 monoclonal antibody 1E10. www.lens.org/lens/patent/147-382-786-055-641 2020. [EPISTEMONIKOS ID: 563468dd70e91a939051abfc89e7d250ead2636a]
References to studies awaiting assessment
ACCORD & ACCORD 2 {published data only}
-
- EUCTR2020-001736-95. ACCORD 2: a multicentre, seamless, phase 2 adaptive randomisation platform study to assess the efficacy and safety of multiple candidate agents for the treatment of COVID 19 in hospitalised patients. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001736-95/GB (first received 23 April 2020). - PMC - PubMed
-
- Wilkinson T, Dixon R, Page C, Carroll M, Griffiths G, Ho LP, et al. ACCORD: a multicentre, seamless, phase 2 adaptive randomisation platform study to assess the efficacy and safety of multiple candidate agents for the treatment of COVID-19 in hospitalised patients: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):691. [DOI: 10.1186/s13063-020-04584-9] - DOI - PMC - PubMed
ACOVACT {published data only}
-
- Euctr2020-001302-30/AT. A multicenter, randomized, active controlled, open label, platform trial on the efficacy and safety of experimental therapeutics for patients with a lung disease caused by coronavirus infection. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001302-30/AT (first received 9 April 2020).
-
- NCT04351724. Austrian corona virus adaptive clinical trial (COVID-19). clinicaltrials.gov/ct2/show/NCT04351724 (first received 2 March 2021).
ACTIV‐1 IM {published data only}
-
- NCT04593940. Immune modulators for treating COVID-19. clinicaltrials.gov/ct2/show/NCT04593940 (first received 20 October 2020).
ACTT‐4 {published data only}
-
- NCT04640168. Adaptive COVID-19 treatment trial 4 (ACTT-4). clinicaltrials.gov/ct2/show/NCT04640168 (first received 23 November 2020).
ANTICOV {published data only}
-
- PACTR202006537901307. An open-label, multicentre, randomised, adaptive platform trial of the safety and efficacy of several therapies, including antiviral therapies, versus control in mild/moderate cases of COVID-19. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=12150 (first received 24 June 2020).
ARCO‐Home {published data only}
-
- EU/CTR/2020-001528-32. Adaptive randomised trial for therapy of corona virus disease 2019 at home with oral antivirals. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001528-32/IT (first received 24 June 2020).
BEAT COVID‐19 {published data only}ACTRN12620000566932
-
- ACTRN12620000566932. Randomised clinical trial of interventions for the treatment of COVID-19 in the community setting for high risk older people. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379801&isRe... (first received 10 May 2020).
BET‐A (ACTIV‐5) {published data only}
-
- NCT04583956. Big effect trial (BET-A) for the treatment of COVID-19. www.clinicaltrials.gov/ct2/show/NCT04583956 (first received 12 October 2020).
BET‐B (ACTIV‐5) {published data only}
-
- NCT04583969. Big effect trial (BET-B) for the treatment of COVID-19. www.clinicaltrials.gov/ct2/show/NCT04583969 (first received 12 October 2020).
CATALYST {published data only}
-
- ISRCTN40580903. Which treatment could lessen the severity of a coronavirus infection when compared with usual care in an NHS setting? www.isrctn.com/ISRCTN40580903 (first received 14 May 2020).
CCAP {published data only}
-
- NCT04345289. Efficacy and safety of novel treatment options for adults with COVID-19 pneumonia. clinicaltrials.gov/ct2/show/NCT04345289 (first received 14 April 2020).
COLHEART‐19 {published data only}
-
- NCT04355143. Colchicine to reduce myocardial injury in COVID-19 (COLHEART-19). clinicaltrials.gov/ct2/show/NCT04355143 (first received 12 April 2020).
COPPS {published data only}
-
- NCT04662086. COVID-19 outpatient pragmatic platform study (COPPS) - master protocol. clinicaltrials.gov/ct2/show/NCT04662086 (first received 10 December 2020).
CORIMUNO {published data only}
-
- NCT04324047. Cohort multiple randomized controlled trials open-label of immune modulatory drugs and other treatments in COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04324047 (first received 27 March 2020).
COVID MED {published data only}
-
- NCT04328012. Comparison of therapeutics for hospitalized patients infected with SARS-CoV-2 in a pragmatic adaptive randomized clinical trial during the COVID-19 pandemic (COVID MED Trial). clinicaltrials.gov/show/NCT04328012 (first received 31 March 2020).
DEFINE {published data only}
-
- NCT04473053. Rapid experimental medicine for COVID-19. clinicaltrials.gov/ct2/show/NCT04473053 (first received 16 July 2020).
EU SolidAct {published data only}
-
- NCT04891133. EU SolidAct: an adaptive pandemic and emerging infection platform trial. clinicaltrials.gov/ct2/show/NCT04891133.
I‐SPY {published data only}
-
- NCT04488081. I-SPY COVID-19 trial: an adaptive platform trial for critically ill patients. clinicaltrials.gov/ct2/show/NCT04488081 (first received 27 July 2020).
NCT04359095 {published data only}
-
- NCT04359095. Effectiveness and safety of medical treatment for SARS-CoV-2 (COVID-19) in Colombia. clinicaltrials.gov/ct2/show/NCT04359095 (first received 24 April 2020).
NCT04590586 {published data only}
-
- NCT04590586. Study of multiple candidate agents for the treatment of COVID-19 in hospitalized patients. clinicaltrials.gov/ct2/show/NCT04590586 (first received 19 October 2020).
O’Brien 2021 {published data only}
-
- O’Brien M, Forleo-Neto E, Sarkar N, Isa F, Hou P, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination in early SARS-CoV-2 infection. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.06.14.21258569] - DOI
PaTS‐COVID {published data only}
-
- NCT04703608. Prevention and treatment for COVID -19 (severe acute respiratory syndrome coronavirus 2 SARS-CoV-2) associated severe pneumonia in the Gambia (PaTS-COVID). clinicaltrials.gov/ct2/show/NCT04703608 (first received 11 January 2021).
PRINCIPLE {published data only}ISRCTN86534580
-
- ISRCTN86534580. A trial evaluating treatments for suspected coronavirus infection in people aged 50 years and above with pre-existing conditions and those aged 65 years and above. www.isrctn.com/ISRCTN86534580 (first received 20 March 2020).
PROTECT‐Surg {published data only}
-
- EUCTR2020-001448-24-GB. Preventing pulmonary complications in surgical patients at risk of COVID-19. clinicaltrialsregister.eu/ctr-search/trial/2020-001448-24/GB (first received 17 April 2020).
-
- NCT04386070. Preventing pulmonary complications in surgical patients at risk of COVID-19. clinicaltrials.gov/ct2/show/NCT04386070 (first received 13 May 2020).
REMAP‐CAP {published data only}
-
- Angus DC, Berry S, Lewis RJ, Al-Beidh F, Arabi Y, Van Bentum-Puijk W, et al. The randomized embedded multifactorial adaptive platform for community-acquired pneumonia (REMAP-CAP) study: rationale and design. Annals of the American Thoracic Society 2020;17(7):879-91. [DOI: 10.1513/AnnalsATS.202003-192SD] - DOI - PMC - PubMed
-
- EUCTR2015-002340-14-NL. Adaptive trial in severe pneumonia (REMAP-CAP). clinicaltrialsregister.eu/ctr-search/trial/2015-002340-14/NL (first received 13 July 2015).
-
- ISRCTN67000769. An international platform trial for severely ill patients with community-acquired pneumonia or COVID-19. www.isrctn.com/ISRCTN67000769 (first received 9 July 2020).
-
- NCT02735707. Randomized, embedded, multifactorial adaptive platform trial for community- acquired pneumonia (REMAP-CAP). clinicaltrials.gov/ct2/show/NCT02735707 (first received 13 April 2016).
SOLIDARITY {published data only}
-
- ISRCTN83971151. SOLIDARITY: an international randomized controlled trial to evaluate non-licensed COVID-19 treatments in addition to standard of care among hospitalised patients. www.isrctn.com/ISRCTN83971151 (first received 25 March 2020).
-
- NCT04330690. Treatments for COVID-19: Canadian arm of the SOLIDARITY trial. clinicaltrials.gov/ct2/show/NCT04330690 (first received 1 April 2020).
-
- NCT04575064. An International randomized trial of additional treatments for COVID-19 in hospitalized patients who are all receiving the local standard of care - WHO-SOLIDARITY-Germany. clinicaltrials.gov/ct2/show/NCT04575064 (first received 5 October 2020).
-
- Soto A, Quinones-Laveriano DM, Garcia PJ, Gotuzzo E, Henao-Restrepo AM. Rapid responses to the COVID-19 pandemic through science and global collaboration: the solidarity clinical trial. Revista Peruana de Medicina Experimental y Salud Publica 2020;37(2):356-60. [DOI: 10.17843/rpmesp.2020.372.5546] - DOI - PubMed
SWISSPED‐RECOVERY {published data only}
-
- NCT04826588. Randomised evaluation of COVID-19 therapy (RECOVERY) in children with PIMS-TS in Switzerland (SWISSPED-RECOVERY). clinicaltrials.gov/ct2/show/NCT04826588.
TACTIC‐E {published data only}
-
- NCT04393246. Multi-arm therapeutic study in pre-ICU patients admitted with COVID-19 - experimental drugs and mechanisms. clinicaltrials.gov/ct2/show/NCT04393246 (first received 19 May 2020).
TACTIC‐R {published data only}
-
- Kulkarni S, Fisk M, Kostapanos M, Banham-Hall E, Bond S, Hernan-Sancho E, et al. Repurposed immunomodulatory drugs for COVID-19 in pre-ICU patients - multi-arm therapeutic study in pre-ICU patients admitted with COVID-19 - repurposed drugs (TACTIC-R): a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):626. [DOI: 10.1186/s13063-020-04535-4] - DOI - PMC - PubMed
-
- NCT04390464. Multi-arm therapeutic study in pre-ICU patients admitted with COVID-19 - repurposed drugs (TACTIC-R). clinicaltrials.gov/ct2/show/NCT04390464 (first received 15 May 2020).
TOGETHER‐3 {published data only}
-
- PACTR202007700757139. TOGETHER 3 trial. COVID-19. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=12194 (first received 14 July 2020).
VIRCO {published data only}
-
- NCT04445467. An adaptive clinical trial of antivirals for COVID-19 infection (VIRCO). clinicaltrials.gov/ct2/show/NCT04445467 (first received 24 June 2020).
References to ongoing studies
ACTIV‐2 {published data only}
-
- NCT04518410. ACTIV-2: a study for outpatients with COVID-19. clinicaltrials.gov/ct2/show/NCT04518410 (first received 19 August 2020).
AGILE {published data only}
-
- EUCTR2020-001860-27. AGILE: seamless phase I/IIa platform for the rapid evaluation of candidates for COVID-19 treatment. A randomised phase I/II study to determine the safety and effectiveness of multiple drugs for the treatment of COVID-19. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001860-27/GB (first received 17 April 2020).
-
- Griffiths G, Fitzgerald R, Jaki T, Corkhill A, Marwood E, Reynolds H, et al. AGILE-ACCORD: a randomized, multicentre, seamless, adaptive phase I/II platform study to determine the optimal dose, safety and efficacy of multiple candidate agents for the treatment of COVID-19: a structured summary of a study protocol for a randomised platform trial. Trials 2020;21(1):544. [DOI: 10.1186/s13063-020-04473-1] - DOI - PMC - PubMed
DISCOVERY {published data only}
-
- NCT04315948. Trial of treatments for COVID-19 in hospitalised adults (DisCoVeRy). clinicaltrials.gov/ct2/show/NCT04315948 (first received 20 March 2020).
EUDRACT2020‐003401‐60 {published data only}
-
- EUDRACT2020-003401-60. A phase 2/3, randomized, parallel-group, placebo-controlled, double-blind study to evaluate the efficacy and safety of CT-P59 in combination with standard of care in hospitalized patients with SARS-CoV-2 Iinfection (COVID19). www.clinicaltrialsregister.eu/ctr-search/trial/2020-003401-60/IT.
NCT04411628 {published data only}
-
- NCT04411628. A study of LY3819253 (LY-CoV555) in participants hospitalized for COVID-19. clinicaltrials.gov/ct2/show/NCT04411628 (first received 2 June 2020).
NCT04426695 {published data only}
-
- NCT04426695. Safety, tolerability, and efficacy of anti-Spike (S) SARS-CoV-2 monoclonal antibodies for hospitalized adult patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04426695 (first received 11 June 2020).
NCT04551898 {published data only}
-
- NCT04551898. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibody BGB-DXP593 in participants with mild-to-moderate coronavirus disease 2019 (COVID-19). clinicaltrials.gov/ct2/show/NCT04551898 (first received 16 September 2020).
NCT04584697 {published data only}
-
- NCT04584697. Study to evaluate the safety, pharmacokinetics and efficacy of STI-2020 (COVI-AMG™) in outpatients with COVID-19. clinicaltrials.gov/ct2/show/NCT04584697 (first received 14 October 2020).
NCT04593641 {published data only}
-
- NCT04593641. A pilot phase 1, randomized, double-blind, placebo-controlled, parallel group, single ascending dose study to evaluate the safety, tolerability and virology of CT-P59 in patient with mild symptoms of SARS-CoV-2 infection. clinicaltrials.gov/ct2/show/NCT04593641 (first received 20 October 2020).
NCT04627584 {published data only}
-
- NCT04627584. A phase 2 clinical trial of MW33 injection to evaluate the efficacy and safety in patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04627584 (first received 13 November 2020).
NCT04631666 {published data only}
-
- NCT04631666. COVID-19 - administration of the SARS-CoV-2-neutralizing monoclonal antibody DZIF-10c (infusion). clinicaltrials.gov/ct2/show/NCT04631666 (first received 17 November 2020).
NCT04631705 {published data only}
-
- NCT04631705. COVID-19 - administration of the SARS-CoV-2-neutralizing monoclonal antibody DZIF-10c (inhalation). clinicaltrials.gov/ct2/show/NCT04631705 (first received 17 November 2020).
NCT04634409 {published data only}
-
- NCT04634409. A study of immune system proteins in participants with mild to moderate COVID-19 Illness. clinicaltrials.gov/ct2/show/NCT04634409 (first received 18 November 2020).
NCT04644120 {published data only}
-
- NCT04644120. Study to assess adverse events and how intravenous (IV) ABBV-47D11 moves through the body of adult participants hospitalized with coronavirus disease 2019 (COVID-19). clinicaltrials.gov/ct2/show/NCT04644120 (first received 25 November 2020).
NCT04644185 {published data only}
-
- NCT04644185. The efficacy and safety of SCTA01 in hospitalized patients with severe COVID-19. clinicaltrials.gov/ct2/show/NCT04644185 (first received 25 November 2020).
NCT04649515 {published data only}
-
- NCT04649515. Efficacy and safety of TY027, a treatment for COVID-19, in humans. clinicaltrials.gov/ct2/show/NCT04649515 (first received 2 December 2020).
NCT04666441 {published data only}
-
- NCT04666441. A study to assess the virologic efficacy of REGN10933+REGN10987 across different dose regimens in adult outpatients with SARS-CoV-2 infection. clinicaltrials.gov/ct2/show/NCT04666441 (first received 14 December 2020).
NCT04674566 {published data only}
-
- Evaluation of safety and tolerability of COR-101 in hospitalized patients with moderate to severe COVID-19. clinicaltrials.gov/ct2/show/NCT04674566 (first received 19 December 2020). [NCT04748588]
NCT04683328 {published data only}
-
- NCT04683328. The safety and efficacy of SCTA01 against COVID-19 in patients admitted to high dependence or intensive care. clinicaltrials.gov/ct2/show/NCT04683328 (first received 24 December 2020).
NCT04709328 {published data only}
-
- NCT04709328. An adaptive, randomized, double-blinded, placebo-controlled trial to evaluate SCTA01 in COVID-19. clinicaltrials.gov/ct2/show/NCT04709328 (first received 14 January 2021).
NCT04723394 {published data only}
-
- NCT04723394. Phase III study of AZD7442 for treatment of COVID-19 in outpatient adults. clinicaltrials.gov/ct2/show/NCT04723394 (first received 25 January 2021).
NCT04734860 {published data only}
-
- NCT04734860. Study to evaluate a single dose of STI-2020 (COVI-AMG™) in adults with mild COVID-19 symptoms. clinicaltrials.gov/ct2/show/NCT04734860 (first received 2 February 2021).
NCT04748588 {published data only}
-
- NCT04748588. Treatment of nosocomial COVID-19. clinicaltrials.gov/ct2/show/NCT04748588 (first received 10 February 2021).
NCT04770467 {published data only}
-
- NCT04770467. A safety and efficacy study of human monoclonal antibodies, BRII-196 and BRII-198 for the treatment of patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04770467 (first received 25 February 2021). [CLINICALTRIALS.GOV: NCT04770467]
NCT04771351 {published data only}
-
- Study to evaluate a single dose of STI-2020 (COVI-AMG) in hospitalized adults with COVID-19. www.clinicaltrials.gov/ct2/show/NCT04771351 (first received 25 February 2021). [CLINICALTRIALS.GOV: NCT04771351]
NCT04779879 {published data only}
-
- Safety, tolerability and pharmacokinetics of second generation VIR-7831 material in non-hospitalized participants with mild to moderate COVID-19 (COMET-PEAK). clinicaltrials.gov/ct2/show/NCT04779879 (first received 3 March 2021).
NCT04780321 {published data only}
-
- JS016 (anti-SARS-CoV-2 monoclonal antibody) with mild and moderate COVID-19 or SARS-CoV-2 asymptomatic infection subjects. clinicaltrials.gov/ct2/show/NCT04780321 (first received 3 March 2021).
NCT04787211 {published data only}
-
- NCT04787211. A phase 2 study of human monoclonal antibodies, BRII-196 and BRII-198. clinicaltrials.gov/ct2/show/NCT04787211.
NCT04796402 {published data only}
-
- NCT04796402. A study to assess if a medicine called bamlanivimab is safe and effective in reducing hospitalization due to COVID-19 (B-EPIC). clinicaltrials.gov/ct2/show/NCT04796402.
NCT04805671 {published data only}
-
- NCT04805671. Evaluation of ADG20 for the treatment of mild or moderate COVID-19 (STAMP). clinicaltrials.gov/ct2/show/NCT04805671.
NCT04822701 {published data only}
-
- NCT04822701. A study to test BI 767551 in people with mild to moderate symptoms of COVID-19. clinicaltrials.gov/ct2/show/NCT04822701.
NCT04840459 {published data only}
-
- NCT04840459. Use of monoclonal antibodies for the treatment of mild to moderate COVID-19 in non-hospitalized setting. clinicaltrials.gov/ct2/show/NCT04840459.
NCT04900428 {published data only}
-
- NCT04900428. Study to evaluate a single intranasal dose of STI-2099 (COVI-DROPS™) in outpatient adults with COVID-19 (UK). clinicaltrials.gov/ct2/show/NCT04900428.
NCT04913675 {published data only}
-
- NCT04913675. Intramuscular VIR-7831 (sotrovimab) for mild/moderate COVID-19. clinicaltrials.gov/ct2/show/NCT04913675.
NCT04952805 {published data only}
-
- NCT04952805. Clinical trial to select the dose and evaluate safety and efficacy of MAD0004J08 monoclonal antibody in adult patients with recently diagnosed asymptomatic to moderately severe COVID-19. ClinicalTrials.gov 2021.
OPTIMISE‐C19 {published data only}
-
- NCT04790786. UPMC OPTIMISE-C19 Trial, a COVID-19 Study (OPTIMISE-C19). clinicaltrials.gov/ct2/show/NCT04790786.
Additional references
Antibody Society 2020
-
- Reichert J. FDA approves inmazeb for ebola virus infection. www.antibodysociety.org/tag/approved-antibodies (accessed 5 November 2020).
Arvin 2020
AstraZeneca 2020
-
- AstraZeneca. AstraZeneca’s COVID-19 vaccine authorised for emergency supply in the UK. www.astrazeneca.com/media-centre/press-releases/2020/astrazenecas-covid-... (accessed 12 January 2021).
Bayer 2019
Beigel 2020
Buitrago‐Garcia 2020
Buszko 2020
Catanzaro 2020
Cathcart 2021
-
- Cathcart AL, Havenar-Daughton C, Lempp FA, Ma D, Schmid M, Agostini ML, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. [DOI: 10.1101/2021.03.09.434607] - DOI
CDC 2020
-
- Centers for Disease Control and Prevention. COVIDView - a weekly surveillance summary of U.S. COVID-19 activity (Key updates for week 43). www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html (accessed 12 January 2020).
Chen 2020
Cherian 2021
Chinese Antibody Society 2020
-
- Chinese Antibody Society. chineseantibody.org (accessed 9 November 2020).
Choi 2020
Clark 2020
Cochrane LSR
-
- Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode. community.cochrane.org/review-production/production-resources/living-sys... (accessed 2 November 2020).
COMET 2020
-
- Core outcome set developers’ response to COVID-19. www.comet-initiative.org/Studies/Details/1538 (accessed 2 November 2020).
Copin 2021
-
- Copin R, Baum A, Wloga E, Pascal KE, Giordano S, Fulton BO, et al. In vitro and in vivo preclinical studies predict REGEN-COV protection against emergence of viral escape in humans. bioRxiv. [DOI: 10.1101/2021.03.10.434834] - DOI
Covidence [Computer program]
-
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, 2021. Available at covidence.org. [DOI: 10.1016/S1470-2045(20)30096-6] - DOI
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Eldridge 2021
-
- Eldridge S, Campbell M, Campbell M, Dahota A, Giraudeau B, Giraudeau B, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0). Additional considerations for cluster-randomized trials (RoB 2 CRT). https://www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-cluster-rando... (accessed June 11, 2021).
Eli Lilly and Co 2020
-
- Eli Lilly and Company. Lilly begins world's first study of a potential COVID-19 antibody treatment in humans [press release]. investor.lilly.com/news-releases/news-release-details/lilly-begins-world... (accessed 5 November 2020).
Endnote X9 [Computer program]
-
- EndNote X9. EndNote. Clarivate, 2013.
FDA 2021a
-
- US Food and Drug Administration. COVID-19 vaccines. www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019... (accessed 12 January 2021).
FDA 2021b
-
- US Food and Drug Administration. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-... (accessed 27 April 2021).
FDA 2021c
-
- US Food and Drug Administration. Emergency use authorization. www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and....
Ghosn 2021
Glaunsinger 2020
-
- Glaunsinger B. Lecture 2: "Coronavirus biology", from the course "COVID-19, SARS-CoV-2 and the pandemic" [video]. biology.mit.edu/undergraduate/current-students/subject-offerings/covid-1... (accessed 4 November 2020).
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Higgins 2003
Higgins 2021a
-
- Higgins JP Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021b
-
- Higgins JP, Eldridge S, Li T. Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021c
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Hoffmann 2020
Hoffmann 2021a
Hoffmann 2021b
Huang 2020
Ioannidis 2020
Johns Hopkins 2021
-
- Johns Hopkins University & Medicine. Mortality analysis. coronavirus.jhu.edu/data/mortality (accessed 11 March 2021).
Kaplon 2020
Kemp 2021
Lamontagne 2020
Lauer 2020
Lee 2020
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Li 2020
-
- Li T, Higgins JP, Deeks JJ. Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Liang 2020
Liu 2014
Lu 2020a
Lu 2020b
Madhi 2021
Mansourabadi 2020
Marovich 2020
-
- Marovich M, Mascola JR, Cohen MS. Monoclonal antibodies for prevention and treatment of COVID-19. JAMA 2020;324(2):131-2. - PubMed
Marshall 2020
Marston 2018
McCallum 2021
McGuinness 2020
Microsoft 2018 [Computer program]
-
- Mircosoft Excel. Microsoft Corporation. Microsoft Corporation, 2018. office.microsoft.com/excel.
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. - PubMed
Monin‐Aldama 2021
-
- Monin-Aldama L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Molino del Barrio I, Alaguthurai T, et al. Interim results of the safety and immune-efficacy of 1 versus 2 doses of COVID-19 vaccine BNT162b2 for cancer patients in the context of the UK vaccine priority guidelines. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.03.17.21253131] - DOI
Muik 2021
National COVID‐19 Clinical Evidence Taskforce 2020
-
- National COVID-19 Clinical Evidence Taskforce. Caring for people with COVID-19: supporting Australia’s healthcare professionals with continually updated, evidence-based clinical guidelines. covid19evidence.net.au/#living-guidelines (accessed 7 December 2020).
NCT04427501
-
- NCT04427501. A randomized, double-blind, placebo-controlled, phase 2/3 study to evaluate the efficacy and safety of LY3819253 and LY3832479 in participants with mild to moderate COVID-19 illness. clinicaltrials.gov/ct2/show/NCT04427501 (first received 11 June 2020).
NIH 2020
-
- National Institutes of Health. COVID-19 treatment guidelines - therapeutic management of patients with COVID-19 (version last updated on 9 October 2020). www.covid19treatmentguidelines.nih.gov/therapeutic-management (accessed 6 November 2020).
NIH 2021
-
- National Institutes of Health. NIH-sponsored ACTIV-3 clinical trial closes enrollment into two sub-studies. Available from: https://www.nih.gov/news-events/news-releases/nih-sponsored-activ-3-clin... (accessed 29.07.2021).
Ou 2020
Page 2021
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesiss. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Parmar 1998
Piechotta 2020
-
- Piechotta V, Valk SJ, Chai KL, Wood EM, Lamikanra A, Kimber C, et al. Safety and effectiveness of convalescent plasma or hyperimmune globulin for people with COVID-19: a rapid review. osf.io/dwf53 2020. [DOI: 10.17605/OSF.IO/DWF53] - DOI - PMC - PubMed
Piechotta 2021
-
- Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, Dorando E, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013600. [DOI: 10.1002/14651858.CD013600.pub4] - DOI - PMC - PubMed
Planas 2021
-
- Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of infectious SARS-CoV-2 variant B.1.617.2 to monoclonal antibodies and sera from convalescent and vaccinated individuals. bioRxiv 2021. [DOI: 10.1101/2021.05.26.445838] - DOI
RECOVERY 2020
-
- Randomised Evaluation of COVID-19 Therapy. www.recoverytrial.net (accessed 16 November 2020). [CT.GOV: NCT04381936]
REMAP‐CAP 2020
-
- A randomised, embedded, multi-factorial, adaptive platform trial for community-acquired pneumonia. www.remapcap.org (accessed 16 November 2020). [CT.GOV: NCT02735707]
RevMan Web 2020 [Computer program]
-
- Cochrane Collaboration Review Manager Web (RevMan Web). Version 1.22.0. Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Ryu 2021
Santesso 2020
Schünemann 2021
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ,et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Skoetz 2020
-
- Skoetz N, Goldkuhle M, Van Dalen EC, Akl EA, Trivella M, Mustafa RA, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and Evidence Profiles. Journal of Clinical Epidemiology 2020;118:124-31. [DOI: 10.1016/j.jclinepi.2019.10.015] - DOI - PubMed
Starr 2021
-
- Starr TN, Czudnochowski N, Zatta F, Park YJ, Liu Z, Addetia A, et al. Antibodies to the SARS-CoV-2 receptor-binding domain that maximize breadth and resistance to viral escape. bioRxiv [Preprint]. [DOI: 10.1101/2021.04.06.438709] - DOI
Sterne 2019
Struyf 2020
-
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] - DOI - PMC - PubMed
Tang 2020
Tierney 2007
Tolouian 2020
-
- Tolouian R, Vahed SZ, Ghiyasvand S, Tolouian A, Ardalan M. COVID-19 interactions with angiotensin-converting enzyme 2 (ACE2) and the kinin system; looking at a potential treatment. Journal of Renal Injury Prevention 2020;9(2):e19. [DOI: 10.34172/jrip.2020.19] - DOI
Van de Veerdonk 2020a
-
- Van de Veerdonk F, Netea MG, Van Deuren M, Van der Meer JW, De Mast Q, Bruggemann R, et al. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach. Preprints.org 2020. [DOI: 10.20944/preprints202004.0023.v1] - DOI
Van de Veerdonk 2020b
Volz 2020
-
- Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L, et al. Transmission of SARS-CoV-2 lineage B.1.1.7 in England: Insights from linking epidemiological and genetic data. medRxiv [Preprint]. [DOI: 10.1101/2020.12.30.20249034] - DOI
Wang 2021
Wang 2021b
WHO/Cochrane 2020
-
- WHO/Cochrane. COVID-NMA Initiative - a living mapping and living systematic review of COVID-19 trials. covid-nma.com (accessed 6 November 2020).
WHO 2007
-
- World Health Organization. Cumulative number of reported probable cases of SARS. www.who.int/csr/sars/country/2003_07_11/en (accessed 13 April 2020).
WHO 2019
-
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). www.who.int/emergencies/mers-cov/en (accessed 13 April 2020).
WHO 2020a
-
- World Health Organization. Report of the WHO‐China Joint Mission on coronavirus disease 2019 (COVID‐19). www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-... (accessed 6 November 2020).
WHO 2020b
-
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. covid19.who.int (accessed 11 June 2020).
WHO 2020c
-
- World Health Organization. Estimating mortality from COVID-19 - scientific brief. www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1 (accessed 2 November 2020).
WHO 2020d
-
- World Health Organization. SARS-CoV-2 mink-associated variant strain – Denmark. www.who.int/csr/don/06-november-2020-mink-associated-sars-cov2-denmark/en (accessed 9 November 2020).
WHO 2020e
-
- World Health Organization. Corticosteroids for COVID-19 - living guidance 2 September 2020. www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (accessed 5 November 2020).
WHO 2020f
-
- World Health Organization. SARS-CoV-2 variants. www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/ 2020. - PubMed
WHO 2021a
-
- World Health Organisation. WHO validates Sinovac COVID-19 vaccine for emergency use and issues interim policy recommendations. https://www.who.int/news/item/01-06-2021-who-validates-sinovac-covid-19-....
WHO 2021b
-
- World Health Organization. Weekly epidemiological update on COVID-19 - 27 April 2021. www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-1... (accessed 3 May 2021).
WHO 2021c
-
- World Health Organization. COVID-19 Clinical management - living guidance. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (last accessed 08.07.2021) 2021. [WHO/2019-nCoV/clinical/2021.1]
Widera 2021
-
- Widera M, Wilhelm A, Hoehl S, Pallas C, Kohmer N, Wolf T, et al. Bamlanivimab does not neutralize two SARS-CoV-2 variants carrying E484K in vitro. medRxiv [Preprint] (accessed 16 March 2021).
Williamson 2020
Wu 2020
Yang 2020
Zhou 2021
References to other published versions of this review
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

