Nivolumab Versus Gemcitabine or Pegylated Liposomal Doxorubicin for Patients With Platinum-Resistant Ovarian Cancer: Open-Label, Randomized Trial in Japan (NINJA)
- PMID: 34473544
- PMCID: PMC8601279
- DOI: 10.1200/JCO.21.00334
Nivolumab Versus Gemcitabine or Pegylated Liposomal Doxorubicin for Patients With Platinum-Resistant Ovarian Cancer: Open-Label, Randomized Trial in Japan (NINJA)
Abstract
Purpose: This phase III, multicenter, randomized, open-label study investigated the efficacy and safety of nivolumab versus chemotherapy (gemcitabine [GEM] or pegylated liposomal doxorubicin [PLD]) in patients with platinum-resistant ovarian cancer.
Materials and methods: Eligible patients had platinum-resistant epithelial ovarian cancer, received ≤ 1 regimen after diagnosis of resistance, and had an Eastern Cooperative Oncology Group performance score of ≤ 1. Patients were randomly assigned 1:1 to nivolumab (240 mg once every 2 weeks [as one cycle]) or chemotherapy (GEM 1000 mg/m2 for 30 minutes [once on days 1, 8, and 15] followed by a week's rest [as one cycle], or PLD 50 mg/m2 once every 4 weeks [as one cycle]). The primary outcome was overall survival (OS). Secondary outcomes included progression-free survival (PFS), overall response rate, duration of response, and safety.
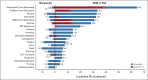
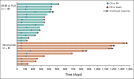
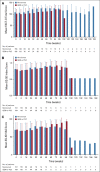
Results: Patients (n = 316) were randomly assigned to nivolumab (n = 157) or GEM or PLD (n = 159) between October 2015 and December 2017. Median OS was 10.1 (95% CI, 8.3 to 14.1) and 12.1 (95% CI, 9.3 to 15.3) months with nivolumab and GEM or PLD, respectively (hazard ratio, 1.0; 95% CI, 0.8 to 1.3; P = .808). Median PFS was 2.0 (95% CI, 1.9 to 2.2) and 3.8 (95% CI, 3.6 to 4.2) months with nivolumab and GEM or PLD, respectively (hazard ratio, 1.5; 95% CI, 1.2 to 1.9; P = .002). There was no statistical difference in overall response rate between groups (7.6% v 13.2%; odds ratio, 0.6; 95% CI, 0.2 to 1.3; P = .191). Median duration of response was numerically longer with nivolumab than GEM or PLD (18.7 v 7.4 months). Fewer treatment-related adverse events were observed with nivolumab versus GEM or PLD (61.5% v 98.1%), with no additional or new safety risks.
Conclusion: Although well-tolerated, nivolumab did not improve OS and showed worse PFS compared with GEM or PLD in patients with platinum-resistant ovarian cancer.
Conflict of interest statement
Figures




Comment in
-
Checkpoint Blockade: Not Yet NINJA Status in Ovarian Cancer.J Clin Oncol. 2021 Nov 20;39(33):3651-3655. doi: 10.1200/JCO.21.01886. Epub 2021 Sep 16. J Clin Oncol. 2021. PMID: 34529504 Free PMC article. No abstract available.
-
Nivolumab Versus Gemcitabine or Pegylated Liposomal Doxorubicin in Patients With Platinum-Resistant Ovarian Cancer.J Clin Oncol. 2022 Feb 10;40(5):522-523. doi: 10.1200/JCO.21.02208. Epub 2021 Dec 10. J Clin Oncol. 2022. PMID: 34890243 No abstract available.
-
Reply to D.-C. Mo et al.J Clin Oncol. 2022 Feb 10;40(5):523-524. doi: 10.1200/JCO.21.02636. Epub 2021 Dec 10. J Clin Oncol. 2022. PMID: 34890244 No abstract available.
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 - PubMed
-
- Ottevanger PB: Ovarian cancer stem cells more questions than answers. Semin Cancer Biol 44:67-71, 2017 - PubMed
-
- Raja FA, Chopra N, Ledermann JA: Optimal first-line treatment in ovarian cancer. Ann Oncol 23:x118-127, 2012. (suppl 10) - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

