AMPK mediates regulation of glomerular volume and podocyte survival
- PMID: 34473647
- PMCID: PMC8525649
- DOI: 10.1172/jci.insight.150004
AMPK mediates regulation of glomerular volume and podocyte survival
Abstract
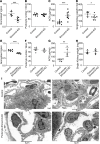
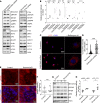
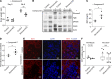
Herein, we report that Shroom3 knockdown, via Fyn inhibition, induced albuminuria with foot process effacement (FPE) without focal segmental glomerulosclerosis (FSGS) or podocytopenia. Interestingly, knockdown mice had reduced podocyte volumes. Human minimal change disease (MCD), where podocyte Fyn inactivation was reported, also showed lower glomerular volumes than FSGS. We hypothesized that lower glomerular volume prevented the progression to podocytopenia. To test this hypothesis, we utilized unilateral and 5/6th nephrectomy models in Shroom3-KD mice. Knockdown mice exhibited less glomerular and podocyte hypertrophy after nephrectomy. FYN-knockdown podocytes had similar reductions in podocyte volume, implying that Fyn was downstream of Shroom3. Using SHROOM3 or FYN knockdown, we confirmed reduced podocyte protein content, along with significantly increased phosphorylated AMPK, a negative regulator of anabolism. AMPK activation resulted from increased cytoplasmic redistribution of LKB1 in podocytes. Inhibition of AMPK abolished the reduction in glomerular volume and induced podocytopenia in mice with FPE, suggesting a protective role for AMPK activation. In agreement with this, treatment of glomerular injury models with AMPK activators restricted glomerular volume, podocytopenia, and progression to FSGS. Glomerular transcriptomes from MCD biopsies also showed significant enrichment of Fyn inactivation and Ampk activation versus FSGS glomeruli. In summary, we demonstrated the important role of AMPK in glomerular volume regulation and podocyte survival. Our data suggest that AMPK activation adaptively regulates glomerular volume to prevent podocytopenia in the context of podocyte injury.
Keywords: Cell Biology; Cytoskeleton; Mouse models; Nephrology; Structural biology.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

