National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2020
- PMID: 34473682
- PMCID: PMC8422873
- DOI: 10.15585/mmwr.mm7035a1
National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2020
Abstract
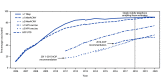
The Advisory Committee on Immunization Practices (ACIP) recommends that adolescents aged 11-12 years routinely receive tetanus, diphtheria, and acellular pertussis (Tdap); meningococcal conjugate (MenACWY); and human papillomavirus (HPV) vaccines. Catch-up vaccination is recommended for hepatitis B (HepB); hepatitis A (HepA); measles, mumps, and rubella (MMR); and varicella (VAR) vaccines for adolescents whose childhood vaccinations are not current. Adolescents are also recommended to receive a booster dose of MenACWY vaccine at age 16 years, and shared clinical decision-making is recommended for the serogroup B meningococcal vaccine (MenB) for persons aged 16-23 years (1). To estimate coverage with recommended vaccines, CDC analyzed data from the 2020 National Immunization Survey-Teen (NIS-Teen) for 20,163 adolescents aged 13-17 years.* Coverage with ≥1 dose of HPV vaccine increased from 71.5% in 2019 to 75.1% in 2020. The percentage of adolescents who were up to date† with HPV vaccination (HPV UTD) increased from 54.2% in 2019 to 58.6% in 2020. Coverage with ≥1 dose of Tdap, ≥1 dose (and among adolescents aged 17 years, ≥2 doses) of MenACWY remained similar to coverage in 2019 (90.1%, 89.3%, and 54.4% respectively). Coverage increased for ≥2 doses of HepA among adolescents aged 13-17 years and ≥1 dose of MenB among adolescents aged 17 years. Adolescents living below the federal poverty level§ had higher HPV vaccination coverage than adolescents living at or above the poverty level. Adolescents living outside a metropolitan statistical area (MSA)¶ had lower coverage with ≥1 MenACWY and ≥1 HPV dose, and a lower proportion being HPV UTD than adolescents in MSA principal cities. In 2020, the COVID-19 pandemic disrupted routine immunization services. Results from the 2020 NIS-Teen reflect adolescent vaccination coverage before the COVID-19 pandemic. The 2020 NIS-Teen data could be used to assess the impact of the COVID-19 pandemic on catch-up vaccination but not on routine adolescent vaccination because adolescents included in the survey were aged ≥13 years, past the age when most routine adolescent vaccines are recommended, and most vaccinations occurred before March 2020. Continued efforts to reach adolescents whose routine medical care has been affected by the COVID-19 pandemic are necessary to protect persons and communities from vaccine-preventable diseases and outbreaks.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- Wodi AP, Ault K, Hunter P, McNally V, Szilagyi PG, Bernstein H. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2021. MMWR Morb Mortal Wkly Rep 2021;70:189–92. 10.15585/mmwr.mm7006a1 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

