A novel angiotensin I-converting enzyme inhibitory peptide derived from the trypsin hydrolysates of salmon bone proteins
- PMID: 34473745
- PMCID: PMC8412326
- DOI: 10.1371/journal.pone.0256595
A novel angiotensin I-converting enzyme inhibitory peptide derived from the trypsin hydrolysates of salmon bone proteins
Abstract
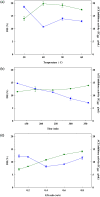
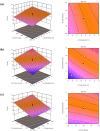
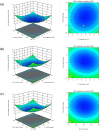
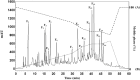
When fish are processed, fish bone becomes a key component of the waste, but to date very few researchers have sought to use fish bone to prepare protein hydrolysates as a means of adding value to the final product. This study, therefore, examines the potential of salmon bone, through an analysis of the benefits of its constituent components, namely fat, moisture, protein, and ash. In particular, the study seeks to optimize the process of enzymatic hydrolysis of salmon bone with trypsin in order to produce angiotensin-I converting enzyme (ACE) inhibitory peptides making use of response surface methodology in combination with central composite design (CCD). Optimum hydrolysis conditions concerning DH (degree of hydrolysis) and ACE-inhibitory activity were initially determined using the response surface model. Having thus determined which of the salmon bone protein hydrolysates (SBPH) offered the greatest level of ACE-inhibitory activity, these SBPH were duly selected to undergo ultrafiltration for further fractionation. It was found that the greatest ACE-inhibitory activity was achieved by the SBPH fraction which had a molecular weight lower than 0.65 kDa. This fraction underwent further purification using RP-HPLC, revealing that the F7 fraction offered the best ACE-inhibitory activity. For ACE inhibition, the ideal peptide in the context of the F7 fraction comprised eight amino acids: Phe-Cys-Leu-Tyr-Glu-Leu-Ala-Arg (FCLYELAR), while analysis of the Lineweaver-Burk plot revealed that the FCLYELAR peptide can serve as an uncompetitive ACE inhibitor. An examination of the molecular docking process showed that the FCLYELAR peptide was primarily able to provide ACE-inhibitory qualities as a consequence of the hydrogen bond interactions taking place between ACE and the peptide. Furthermore, upon isolation form the SBPH, the ACE-inhibitory peptide demonstrated ACE-inhibitory capabilities in vitro, underlining its potential for applications in the food and pharmaceutical sectors.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

