Stem cell-derived neurons reflect features of protein networks, neuropathology, and cognitive outcome of their aged human donors
- PMID: 34473944
- PMCID: PMC8571042
- DOI: 10.1016/j.neuron.2021.08.003
Stem cell-derived neurons reflect features of protein networks, neuropathology, and cognitive outcome of their aged human donors
Abstract
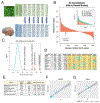
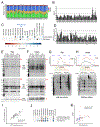
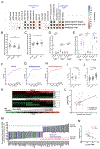
We have generated a controlled and manipulable resource that captures genetic risk for Alzheimer's disease: iPSC lines from 53 individuals coupled with RNA and proteomic profiling of both iPSC-derived neurons and brain tissue of the same individuals. Data collected for each person include genome sequencing, longitudinal cognitive scores, and quantitative neuropathology. The utility of this resource is exemplified here by analyses of neurons derived from these lines, revealing significant associations between specific Aβ and tau species and the levels of plaque and tangle deposition in the brain and, more importantly, with the trajectory of cognitive decline. Proteins and networks are identified that are associated with AD phenotypes in iPSC neurons, and relevant associations are validated in brain. The data presented establish this iPSC collection as a resource for investigating person-specific processes in the brain that can aid in identifying and validating molecular pathways underlying AD.
Keywords: Alzheimer’s; Aβ; LOAD; MAPT; PP1; PRS; genetics; iPSC; neuron; tau.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.J.S. is a director and consultant for Prothena Biosciences.
Figures




References
-
- ABUD EM, RAMIREZ RN, MARTINEZ ES, HEALY LM, NGUYEN CHH, NEWMAN SA, YEROMIN AV, SCARFONE VM, MARSH SE, FIMBRES C, CARAWAY CA, FOTE GM, MADANY AM, AGRAWAL A, KAYED R, GYLYS KH, CAHALAN MD, CUMMINGS BJ, ANTEL JP, MORTAZAVI A, CARSON MJ, POON WW & BLURTON-JONES M 2017. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron, 94, 278–293 e9. - PMC - PubMed
-
- BARNES LL, NELSON JK & REUTER-LORENZ PA 2001. Object-based attention and object working memory: overlapping processes revealed by selective interference effects in humans. Prog Brain Res, 134, 471–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AG056011/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- R01 NS117446/NS/NINDS NIH HHS/United States
- RF1 AG057457/AG/NIA NIH HHS/United States
- R01 AG061798/AG/NIA NIH HHS/United States
- U01 AG046152/AG/NIA NIH HHS/United States
- P01 AG015379/AG/NIA NIH HHS/United States
- RF1 AG057440/AG/NIA NIH HHS/United States
- U01 AG061356/AG/NIA NIH HHS/United States
- U01 AG032984/AG/NIA NIH HHS/United States
- R01 AG030146/AG/NIA NIH HHS/United States
- U01 AG046170/AG/NIA NIH HHS/United States
- R01 AG055909/AG/NIA NIH HHS/United States
- R01 AG061894/AG/NIA NIH HHS/United States
- U01 AG072572/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- R21 MH118576/MH/NIMH NIH HHS/United States
- RF1 NS117446/NS/NINDS NIH HHS/United States
- R01 AG036836/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

