IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice
- PMID: 34473953
- PMCID: PMC8425601
- DOI: 10.1016/j.neuron.2021.06.015
IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice
Abstract
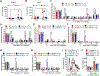
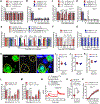
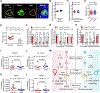
Although sex dimorphism is increasingly recognized as an important factor in pain, female-specific pain signaling is not well studied. Here we report that administration of IL-23 produces mechanical pain (mechanical allodynia) in female but not male mice, and chemotherapy-induced mechanical pain is selectively impaired in female mice lacking Il23 or Il23r. IL-23-induced pain is promoted by estrogen but suppressed by androgen, suggesting an involvement of sex hormones. IL-23 requires C-fiber nociceptors and TRPV1 to produce pain but does not directly activate nociceptor neurons. Notably, IL-23 requires IL-17A release from macrophages to evoke mechanical pain in females. Low-dose IL-17A directly activates nociceptors and induces mechanical pain only in females. Finally, deletion of estrogen receptor subunit α (ERα) in TRPV1+ nociceptors abolishes IL-23- and IL-17-induced pain in females. These findings demonstrate that the IL-23/IL-17A/TRPV1 axis regulates female-specific mechanical pain via neuro-immune interactions. Our study also reveals sex dimorphism at both immune and neuronal levels.
Keywords: IL-17; IL-23; dorsal root ganglion; estrogen receptor α; human; macrophage; mechanical allodynia; nociceptor; nonhuman primate; sex dimorphism.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.-R.J. is a consultant of Boston Scientific and received research grant from the company. These activities are not related to this study. The other authors declare no competing interests.
Figures





Comment in
-
Addressing the gender pain gap.Neuron. 2021 Sep 1;109(17):2641-2642. doi: 10.1016/j.neuron.2021.08.006. Neuron. 2021. PMID: 34473950
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

