Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension
- PMID: 34474396
- PMCID: PMC8408632
- DOI: 10.1016/j.redox.2021.102115
Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension
Abstract
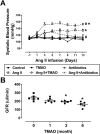
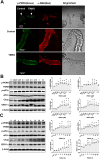
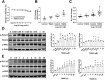
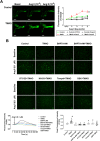
Gut microbiota produce Trimethylamine N-oxide (TMAO) by metabolizing dietary phosphatidylcholine, choline, l-carnitine and betaine. TMAO is implicated in the pathogenesis of chronic kidney disease (CKD), diabetes, obesity and atherosclerosis. We test, whether TMAO augments angiotensin II (Ang II)-induced vasoconstriction and hence promotes Ang II-induced hypertension. Plasma TMAO levels were indeed elevated in hypertensive patients, thus the potential pathways by which TMAO mediates these effects were explored. Ang II (400 ng/kg-1min-1) was chronically infused for 14 days via osmotic minipumps in C57Bl/6 mice. TMAO (1%) or antibiotics were given via drinking water. Vasoconstriction of renal afferent arterioles and mesenteric arteries were assessed by microperfusion and wire myograph, respectively. In Ang II-induced hypertensive mice, TMAO elevated systolic blood pressure and caused vasoconstriction, which was alleviated by antibiotics. TMAO enhanced the Ang II-induced acute pressor responses (12.2 ± 1.9 versus 20.6 ± 1.4 mmHg; P < 0.05) and vasoconstriction (32.3 ± 2.6 versus 55.9 ± 7.0%, P < 0.001). Ang II-induced intracellular Ca2+ release in afferent arterioles (147 ± 7 versus 234 ± 26%; P < 0.001) and mouse vascular smooth muscle cells (VSMC, 123 ± 3 versus 157 ± 9%; P < 0.001) increased by TMAO treatment. Preincubation of VSMC with TMAO activated the PERK/ROS/CaMKII/PLCβ3 pathway. Pharmacological inhibition of PERK, ROS, CaMKII and PLCβ3 impaired the effect of TMAO on Ca2+ release. Thus, TMAO facilitates Ang II-induced vasoconstriction, thereby promoting Ang II-induced hypertension, which involves the PERK/ROS/CaMKII/PLCβ3 axis.
Keywords: Afferent arteriole; Angiotensin II; Blood pressure; Calcium; Trimethylamine N-oxide.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Carlstrom M., Lai E.Y., Ma Z., Steege A., Patzak A., Eriksson U.J., Lundberg J.O., Wilcox C.S., Persson A.E. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin ii via modulation of nitric oxide bioavailability. Hypertension. 2010;56:907–913. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

