Role of Venous Endothelial Cells in Developmental and Pathologic Angiogenesis
- PMID: 34474596
- PMCID: PMC9153651
- DOI: 10.1161/CIRCULATIONAHA.121.054071
Role of Venous Endothelial Cells in Developmental and Pathologic Angiogenesis
Abstract
Background: Angiogenesis is a dynamic process that involves expansion of a preexisting vascular network that can occur in a number of physiological and pathological settings. Despite its importance, the origin of the new angiogenic vasculature is poorly defined. In particular, the primary subtype of endothelial cells (capillary, venous, arterial) driving this process remains undefined.
Methods: Endothelial cells were fate-mapped with the use of genetic markers specific to arterial and capillary cells. In addition, we identified a novel venous endothelial marker gene (Gm5127) and used it to generate inducible venous endothelium-specific Cre and Dre driver mouse lines. Contributions of these various types of endothelial cells to angiogenesis were examined during normal postnatal development and in disease-specific setting.
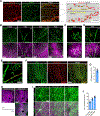
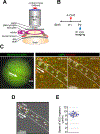
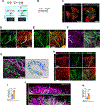
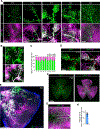
Results: Using a comprehensive set of endothelial subtype-specific inducible reporter mice, including tip, arterial, and venous endothelial reporter lines, we showed that venous endothelial cells are the primary endothelial subtype responsible for the expansion of an angiogenic vascular network. During physiological angiogenesis, venous endothelial cells proliferate, migrating against the blood flow and differentiating into tip, capillary, and arterial endothelial cells of the new vasculature. Using intravital 2-photon imaging, we observed venous endothelial cells migrating against the blood flow to form new blood vessels. Venous endothelial cell migration also plays a key role in pathological angiogenesis. This was observed both in formation of arteriovenous malformations in mice with inducible endothelium-specific Smad4 deletion mice and in pathological vessel growth seen in oxygen-induced retinopathy.
Conclusions: Our studies establish that venous endothelial cells are the primary endothelial subtype responsible for normal expansion of vascular networks, formation of arteriovenous malformations, and pathological angiogenesis. These observations highlight the central role of the venous endothelium in normal development and disease pathogenesis.
Keywords: arteriovenous malformations; cell differentiation; cell lineage; endothelial cells; intravital microscopy; vascular remodeling.
Figures


References
-
- Simons M, Gordon E and Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 2016;17:611–25. - PubMed
-
- Pitulescu ME, Schmidt I, Giaimo BD, Antoine T, Berkenfeld F, Ferrante F, Park H, Ehling M, Biljes D, Rocha SF et al. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nature Cell Biology 2017;19:915–927. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

