Nintedanib in progressive interstitial lung diseases: data from the whole INBUILD trial
- PMID: 34475231
- PMCID: PMC8927709
- DOI: 10.1183/13993003.04538-2020
Nintedanib in progressive interstitial lung diseases: data from the whole INBUILD trial
Abstract
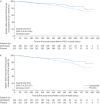
Background: The primary analysis of the INBUILD trial showed that in subjects with progressive fibrosing interstitial lung diseases (ILDs), nintedanib slowed the decline in forced vital capacity (FVC) over 52 weeks. We report the effects of nintedanib on ILD progression over the whole trial.
Methods: Subjects with fibrosing ILDs other than idiopathic pulmonary fibrosis, who had ILD progression within the 24 months before screening despite management deemed appropriate in clinical practice, were randomised to receive nintedanib or placebo. Subjects continued on blinded randomised treatment until all subjects had completed the trial. Over the whole trial, mean±sd exposure to trial medication was 15.6±7.2 and 16.8±5.8 months in the nintedanib and placebo groups, respectively.
Results: In the nintedanib (n=332) and placebo (n=331) groups, respectively, the proportions of subjects who had ILD progression (absolute decline in FVC ≥10% predicted) or died were 40.4% and 54.7% in the overall population (hazard ratio (HR) 0.66, 95% CI 0.53-0.83; p=0.0003) and 43.7% and 55.8% among subjects with a usual interstitial pneumonia (UIP)-like fibrotic pattern on high-resolution computed tomography (HRCT) (HR 0.69, 95% CI 0.53-0.91; p=0.009). In the nintedanib and placebo groups, respectively, the proportions who had an acute exacerbation of ILD or died were 13.9% and 19.6% in the overall population (HR 0.67, 95% CI 0.46-0.98; p=0.04) and 15.0% and 22.8% among subjects with a UIP-like fibrotic pattern on HRCT (HR 0.62, 95% CI 0.39-0.97; p=0.03).
Conclusion: Based on data from the whole INBUILD trial, nintedanib reduced the risk of events indicating ILD progression.
Trial registration: ClinicalTrials.gov NCT02999178.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: K.R. Flaherty reports grants and personal fees from Boehringer Ingelheim and Roche/Genentech; and personal fees from Bellerophon, Blade Therapeutics, Celgene, FibroGen, Respivant, Sanofi Genzyme and Veracyte. Conflict of interest: A.U. Wells reports personal fees from Blade Therapeutics, Boehringer Ingelheim and Roche. Conflict of interest: V. Cottin reports personal fees and nonfinancial support from Actelion and Roche/Promedior; grants, personal fees and nonfinancial support from Boehringer Ingelheim; and personal fees from AstraZeneca, Bayer/MSD, Bristol-Myers Squibb, Celgene, Fibrogen, Galapagos, Galecto, Novartis, Sanofi and Shionogi. Conflict of interest: A. Devaraj reports personal fees from Boehringer Ingelheim, Galapagos, Galecto, GlaxoSmithKline and Roche. Conflict of interest: Y. Inoue reports other from Asahi Kasei, Boehringer Ingelheim, Galapagos, Roche, Savara, Inc., Shionogi and Taiho; and grants from the Japan Agency for Medical Research and Development and Ministry of Health, Labour and Welfare of Japan. Conflict of interest: L. Richeldi reports grants and personal fees from Boehringer Ingelheim and Roche; and personal fees from Asahi Kasei, Biogen, Bristol-Myers Squibb, Celgene, CSL Behring, FibroGen, ImmuneWorks, Nitto, Pliant Therapeutics, Promedior, Respivant and Toray. Conflict of interest: S.L.F. Walsh reports grants and personal fees from Boehringer Ingelheim; and personal fees from Bracco, Galapagos, Open Source Imaging Consortium, Roche and Sanofi. Conflict of interest: M. Kolb reports grants and personal fees from Boehringer Ingelheim, Pieris, Prometic, Roche; personal fees from Algernon, AstraZeneca, GlaxoSmithKline, Indalo and Third Pole, Inc.; and other from the European Respiratory Society. Conflict of interest: D. Koschel reports personal fees and nonfinancial support from Boehringer Ingelheim; and personal fees from Roche. Conflict of interest: T. Moua has nothing to disclose. Conflict of interest: S. Stowasser is an employee of Boehringer Ingelheim International GmbH. Conflict of interest: R-G. Goeldner is an employee of Boehringer Ingelheim Pharma GmbH & Co. KG. Conflict of interest: R. Schlenker-Herceg is an employee of Boehringer Ingelheim Pharmaceuticals, Inc. Conflict of interest: K.K. Brown reports personal fees and nonfinancial support from Boehringer Ingelheim; grants from the National Heart, Lung, and Blood Institute; personal fees from Biogen, Blade Therapeutics, DevPro Biopharma, Dispersol, Galapagos, Galecto, Huitai Biomedicine, Humanetics, Lifemax, Lilly, Pliant, Third Pole Therapeutics and Theravance; and other from the Open Source Imaging Consortium.
Figures



References
-
- Wells AU, Flaherty KR, Brown KK, et al. . Nintedanib in patients with progressive fibrosing interstitial lung diseases – subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med 2020; 8: 453–460. doi:10.1016/S2213-2600(20)30036-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
