Japanese encephalitis virus live attenuated vaccine strains display altered immunogenicity, virulence and genetic diversity
- PMID: 34475404
- PMCID: PMC8413339
- DOI: 10.1038/s41541-021-00371-y
Japanese encephalitis virus live attenuated vaccine strains display altered immunogenicity, virulence and genetic diversity
Abstract
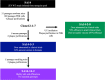
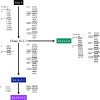
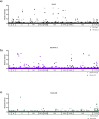
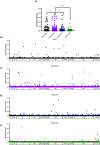
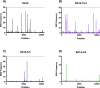
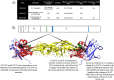
Japanese encephalitis virus (JEV) is the etiological agent of Japanese encephalitis (JE). The most commonly used vaccine used to prevent JE is the live-attenuated strain SA14-14-2, which was generated by serial passage of the wild-type (WT) JEV strain SA14. Two other vaccine candidates, SA14-5-3 and SA14-2-8 were derived from SA14. Both were shown to be attenuated but lacked sufficient immunogenicity to be considered effective vaccines. To better contrast the SA14-14-2 vaccine with its less-immunogenic counterparts, genetic diversity, ribavirin sensitivity, mouse virulence and mouse immunogenicity of the three vaccines were investigated. Next generation sequencing demonstrated that SA14-14-2 was significantly more diverse than both SA14-5-3 and SA14-2-8, and was slightly less diverse than WT SA14. Notably, WT SA14 had unpredictable levels of diversity across its genome whereas SA14-14-2 is highly diverse, but genetic diversity is not random, rather the virus only tolerates variability at certain residues. Using Ribavirin sensitivity in vitro, it was found that SA14-14-2 has a lower fidelity replication complex compared to SA14-5-3 and SA14-2-8. Mouse virulence studies showed that SA14-2-8 was the most virulent of the three vaccine strains while SA14-14-2 had the most favorable combination of safety (virulence) and immunogenicity for all vaccines tested. SA14-14-2 contains genetic diversity and sensitivity to the antiviral Ribavirin similar to WT parent SA14, and this genetic diversity likely explains the (1) differences in genomic sequences reported for SA14-14-2 and (2) the encoding of major attenuation determinants by the viral E protein.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Fischer M, Lindsey N, Staples JE, Hills S. Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbidity Mortal. Wkly. Rep. Recommendations Rep. 2010;59:126. - PubMed
-
- Sohn YM, Tandan JB, Yoksan S, Ji M, Ohrr H. A 5-year follow-up of antibody response in children vaccinated with single dose of live attenuated SA14-14-2 Japanese encephalitis vaccine: Immunogenicity and anamnestic responses. Vaccine. 2008;26:1638–1643. doi: 10.1016/j.vaccine.2008.01.021. - DOI - PubMed
Grants and funding
- None/Gillson Longenbaugh Foundation
- T32 AI 060549/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R21 AI129844/AI/NIAID NIH HHS/United States
- R21 AI 129844/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- T32 AI060549/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

