A Multi-Sensor Mini-Bioreactor to Preselect Silage Inoculants by Tracking Metabolic Activity in situ During Fermentation
- PMID: 34475854
- PMCID: PMC8406527
- DOI: 10.3389/fmicb.2021.673795
A Multi-Sensor Mini-Bioreactor to Preselect Silage Inoculants by Tracking Metabolic Activity in situ During Fermentation
Abstract
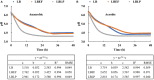
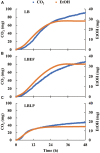
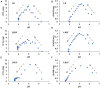
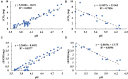
The microbiome in silage may vary substantially from the onset to the completion of fermentation. Improved additives and inoculants are being developed to accelerate the ensiling process, to enhance fermentation quality, and to delay spoilage during feed-out. However, current methods for preselecting and characterizing these amendments are time-consuming and costly. Here, we have developed a multi-sensor mini-bioreactor (MSMB) to track microbial fermentation in situ and additionally presented a mathematical model for the optimal assessment among candidate inoculants based on the Bolza equation, a fundamental formula in optimal control theory. Three sensors [pH, CO2, and ethanol (EtOH)] provided data for assessment, with four additional sensors (O2, gas pressure, temperature, and atmospheric pressure) to monitor/control the fermentation environment. This advanced MSMB is demonstrated with an experimental method for evaluating three typical species of lactic acid bacteria (LAB), Lentilactobacillus buchneri (LB) alone, and LB mixed with Lactiplantibacillus plantarum (LBLP) or with Enterococcus faecium (LBEF), all cultured in De Man, Rogosa, and Sharpe (MRS) broth. The fermentation process was monitored in situ over 48 h with these candidate microbial strains using the MSMB. The experimental results combine acidification characteristics with production of CO2 and EtOH, optimal assessment of the microbes, analysis of the metabolic sensitivity to pH, and partitioning of the contribution of each species to fermentation. These new data demonstrate that the MSMB associated with the novel rapid data-processing method may expedite development of microbial amendments for silage additives.
Keywords: carbon dioxide (CO2); ethanol (EtOH); fermentation; lactic acid bacteria (LAB); metabolic sensitivity; multi-sensor mini-bioreactor (MSMB); pH; silage additive.
Copyright © 2021 Shan, Rosner, Milimonka, Buescher, Lipski, Maack, Berchtold, Wang, Grantz and Sun.
Conflict of interest statement
VR and AM were employed by company ADDCON GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Arasu M. V., Jung M. W., Kim D. H., Park H. S., Ilavenil S., Al-Dhabi N. A., et al. (2015). Identification and phylogenetic characterization of novel Lactobacillus plantarum species and their metabolite profiles in grass silage. Ann. Microbiol. 65 15–25. 10.1007/s13213-014-0830-2 - DOI
-
- Bolsen K. K., Aschbell G., Weinberg Z. G. (1996). Silage fermentation and silage additives – Review. Asian-Australas. J. Anim. Sci. 9 483–493. 10.5713/ajas.1996.483 - DOI
-
- Clarke F. H. (1976). The generalized problem of Bolza. SIAM J. Control Optim. 14 682–699. 10.1137/0314044 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

