Evolving Defect Chemistry of (Pu,Am)O2± x
- PMID: 34476035
- PMCID: PMC8392350
- DOI: 10.1021/acs.jpcc.1c03274
Evolving Defect Chemistry of (Pu,Am)O2± x
Abstract
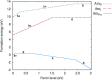
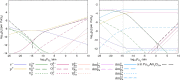
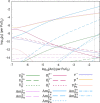
The β decay of 241Pu to 241Am results in a significant ingrowth of Am during the interim storage of PuO2. Consequently, the safe storage of the large stockpiles of separated Pu requires an understanding of how this ingrowth affects the chemistry of PuO2. This work combines density functional theory (DFT) defect energies and empirical potential calculations of vibrational entropies to create a point defect model to predict how the defect chemistry of PuO2 evolves due to the incorporation of Am. The model predicts that Am occupies Pu sites in (Pu,Am)O2±x in either the +III or +IV oxidation state. High temperatures, low oxygen-to-metal (O/M) ratios, or low Am concentrations favor Am in the +III oxidation state. Am (+III) exists in (Pu,Am)O2±x as the negatively charged (AmPu 1-) defect, requiring charge compensation from holes in the valence band, thereby increasing the conductivity of the material compared to Am-free PuO2. Oxygen vacancies take over as the charge compensation mechanism at low O/M ratios. In (Pu,Am)O2±x , hypo- and (negligible) hyperstoichiometry is found to be provided by the doubly charged oxygen vacancy (VO 2+) and singly charged oxygen interstitial (Oi 1-), respectively.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Bailey G.; Bluhm E.; Lyman J.; Mason R.; Paffett M.; Polansky G.; Roberson G. D.; Sherman M.; Veirs K.; Worl L.. Gas Generation from Actinide Oxide Materials, No. LA-13781-MS; Los Alamos National Laboratory: NM (US), 2000; 72.
-
- Peterson V.Reference Computations of Public Dose and Cancer Risk from Airborne Releases of Plutonium. Nuclear Safety Technical Report, 1993.
-
- Chen J. L.; Kaltsoyannis N. Computational Study of Plutonium-Americium Mixed Oxides (Pu0.92Am0.08O2 – x); Water Adsorption on {111}, {110}, and {100} Surfaces. J. Phys. Chem. C 2020, 124, 6646–6658. 10.1021/acs.jpcc.9b11601. - DOI
-
- Osaka M.; Kurosaki K.; Yamanaka S. Oxygen potential of (Pu0.91Am0.09)O2–x. J. Nucl. Mater. 2006, 357, 69–76. 10.1016/j.jnucmat.2006.05.044. - DOI
LinkOut - more resources
Full Text Sources
