Survey of chemotherapy-induced nausea and vomiting in patients with urothelial carcinoma
- PMID: 34476103
- PMCID: PMC8408675
- DOI: 10.3892/mco.2021.2384
Survey of chemotherapy-induced nausea and vomiting in patients with urothelial carcinoma
Abstract
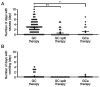
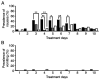
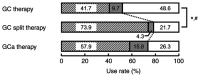
Chemotherapy-induced nausea and vomiting (CINV) can cause anorexia, weight loss and deterioration of patient quality of life. It is one of the most unpleasant adverse effects of chemotherapy treatment regimens. For the optimal treatment of gastrointestinal symptoms during urothelial carcinoma chemotherapy, the present study investigated the association between gastrointestinal symptoms and therapeutic effects of gemcitabine plus platinum [cisplatin (GC) or carboplatin (GCa)] therapies. The incidence and frequency of nausea/vomiting with GC split therapy (gemcitabine, 1,000 mg/m2 on days 1 and 8; split-dose cisplatin, 35 mg/m2 on days 1 and 8; 21-day schedule) and GCa therapy [gemcitabine, 750-1,000 mg/m2 on days 1, 8 and 15; carboplatin, area under the blood concentration-time curve=5 mg min/ml (Calvert formula) on day 2; 28-day schedule] were lower compared with those of GC therapy (gemcitabine, 1,000 mg/m2 on days 1, 8 and 15; single-dose cisplatin 70 mg/m2 on day 2; 28-day schedule). However, no differences in therapeutic outcomes were observed among therapies. GCa therapy, regardless of renal function, and GC split therapy demonstrated significant increases compared with GC therapy in alleviating gastrointestinal symptoms associated with cancer chemotherapy in patients with urothelial carcinoma. Overall, these results suggested that split-dose cisplatin administration or the use of carboplatin instead of cisplatin may be useful in patients who experience CINV without compromising treatment effectiveness.
Keywords: chemotherapy-induced nausea and vomiting; gemcitabine plus carboplatin therapy; gemcitabine plus single-dose cisplatin therapy; gemcitabine plus split-dose cisplatin therapy; urothelial carcinoma.
Copyright © 2020, Spandidos Publications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Comparison of full-dose gemcitabine/cisplatin, dose-reduced gemcitabine/cisplatin, and gemcitabine/carboplatin in real-world patients with advanced urothelial carcinoma.BMC Urol. 2022 Nov 9;22(1):177. doi: 10.1186/s12894-022-01139-9. BMC Urol. 2022. PMID: 36352389 Free PMC article.
-
Chemotherapy for urothelial carcinoma in renal transplantation patients: Initial results from a single center.Mol Clin Oncol. 2015 Nov;3(6):1387-1391. doi: 10.3892/mco.2015.615. Epub 2015 Jul 30. Mol Clin Oncol. 2015. PMID: 26807252 Free PMC article.
-
Eligibility Criteria for Different Platinum-Based Chemotherapy Regimens in Metastatic Urothelial Carcinoma.Cureus. 2024 Aug 9;16(8):e66520. doi: 10.7759/cureus.66520. eCollection 2024 Aug. Cureus. 2024. PMID: 39246966 Free PMC article.
-
Efficacy of the oral neurokinin-1 receptor antagonist aprepitant for nausea and vomiting induced by cisplatin and carboplatin in Japanese patients with gynecological cancer.J Obstet Gynaecol Res. 2017 Oct;43(10):1613-1620. doi: 10.1111/jog.13415. Epub 2017 Jul 10. J Obstet Gynaecol Res. 2017. PMID: 28691209
-
Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Jan 13;1(1):CD009256. doi: 10.1002/14651858.CD009256.pub3. Cochrane Database Syst Rev. 2020. PMID: 31930743 Free PMC article.
Cited by
-
Patient Preferences for First-Line Treatment of Locally Advanced or Metastatic Urothelial Carcinoma: An Application of Multidimensional Thresholding.Patient. 2025 Jan;18(1):77-87. doi: 10.1007/s40271-024-00709-3. Epub 2024 Aug 28. Patient. 2025. PMID: 39198374 Free PMC article.
-
Relationship between nausea and vomiting and physical activity in patients with lung cancer undergoing first chemotherapy.Front Oncol. 2024 Jul 24;14:1396637. doi: 10.3389/fonc.2024.1396637. eCollection 2024. Front Oncol. 2024. PMID: 39114312 Free PMC article.
-
Split-Dose Cisplatin Use, Eligibility Criteria, and Drivers for Treatment Choice in Patients with Locally Advanced or Metastatic Urothelial Carcinoma: Results of a Large International Physician Survey.Cancers (Basel). 2025 Feb 3;17(3):509. doi: 10.3390/cancers17030509. Cancers (Basel). 2025. PMID: 39941876 Free PMC article.
References
-
- Razvi Y, Chan S, McFarlane T, McKenzie E, Zaki P, DeAngelis C, Pidduck W, Bushehri A, Chow E, Jerzak KJ. ASCO, NCCN, MASCC/ESMO: A comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support Care Cancer. 2019;27:87–95. doi: 10.1007/s00520-018-4464-y. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
