Multiple Sclerosis Phenotypes as a Continuum: The Role of Neurologic Reserve
- PMID: 34476126
- PMCID: PMC8382415
- DOI: 10.1212/CPJ.0000000000001045
Multiple Sclerosis Phenotypes as a Continuum: The Role of Neurologic Reserve
Abstract
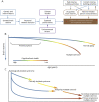
Purpose of review: This review presents the hypothesis that loss of neurologic reserve explains onset of progressive multiple sclerosis (PrMS).
Recent findings: Evidence supporting the separate classification of PrMS and relapsing multiple sclerosis (RMS) is limited and does not explain PrMS or the response of these patients to therapy.
Summary: We argue that multiple sclerosis (MS) progresses along a continuum from RMS to PrMS, with differing levels of neurologic reserve accounting for phenotypic differences. In early MS, inflammation causes brain atrophy with symptoms buffered by neurologic reserve. As brain loss from normal aging and MS continues, reserve is depleted and effects of subclinical MS disease activity and aging are unmasked, manifesting as PrMS. Most therapies show limited benefit in PrMS; patients are older, have fewer inflammatory events, and the effects of aging cause continued loss of neurologic function, even if inflammation is terminated. Loss of neurologic reserve means patients with PrMS cannot recover function, unlike patients with RMS.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures


Comment in
-
Loss of Neurologic Reserve in Progressive Multiple Sclerosis: A Paradigm Shift?Neurol Clin Pract. 2021 Aug;11(4):271-272. doi: 10.1212/CPJ.0000000000001106. Neurol Clin Pract. 2021. PMID: 34484925 Free PMC article. No abstract available.
References
-
- Fisher E, Rudick RA, Simon JH, et al. . Eight-year follow-up study of brain atrophy in patients with MS. Neurology 2002;59:1412–1420. - PubMed
-
- Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol 2014;75:43–49. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
