Repurposing Carvedilol as a Novel Inhibitor of the Trypanosoma cruzi Autophagy Flux That Affects Parasite Replication and Survival
- PMID: 34476220
- PMCID: PMC8406938
- DOI: 10.3389/fcimb.2021.657257
Repurposing Carvedilol as a Novel Inhibitor of the Trypanosoma cruzi Autophagy Flux That Affects Parasite Replication and Survival
Abstract
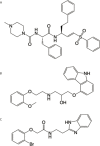
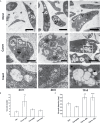
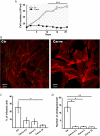
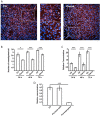
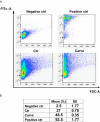
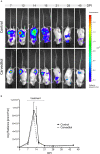
T. cruzi, the causal agent of Chagas disease, is a parasite able to infect different types of host cells and to persist chronically in the tissues of human and animal hosts. These qualities and the lack of an effective treatment for the chronic stage of the disease have contributed to the durability and the spread of the disease around the world. There is an urgent necessity to find new therapies for Chagas disease. Drug repurposing is a promising and cost-saving strategy for finding new drugs for different illnesses. In this work we describe the effect of carvedilol on T. cruzi. This compound, selected by virtual screening, increased the accumulation of immature autophagosomes characterized by lower acidity and hydrolytic properties. As a consequence of this action, the survival of trypomastigotes and the replication of epimastigotes and amastigotes were impaired, resulting in a significant reduction of infection and parasite load. Furthermore, carvedilol reduced the whole-body parasite burden peak in infected mice. In summary, in this work we present a repurposed drug with a significant in vitro and in vivo activity against T. cruzi. These data in addition to other pharmacological properties make carvedilol an attractive lead for Chagas disease treatment.
Keywords: Chagas disease; Trypanosoma cruzi; autophagy flux inhibitor; drug repurposing; mice infection; trypanocidal drugs.
Copyright © 2021 Rivero, Martínez, Novick, Cueto, Salassa, Vanrell, Li, Labriola, Polo, Engman, Clos and Romano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Barclay J. J., Morosi L. G., Vanrell M. C., Trejo E. C., Romano P. S., Carrillo C. (2011). Trypanosoma Cruzi Coexpressing Ornithine Decarboxylase and Green Fluorescence Proteins as a Tool to Study the Role of Polyamines in Chagas Disease Pathology. Enzyme Res. 2011, 1–10. 10.4061/2011/657460 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

