The Beginning of HCN Polymerization: Iminoacetonitrile Formation and Its Implications in Astrochemical Environments
- PMID: 34476321
- PMCID: PMC8397470
- DOI: 10.1021/acsearthspacechem.1c00195
The Beginning of HCN Polymerization: Iminoacetonitrile Formation and Its Implications in Astrochemical Environments
Abstract
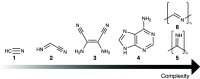
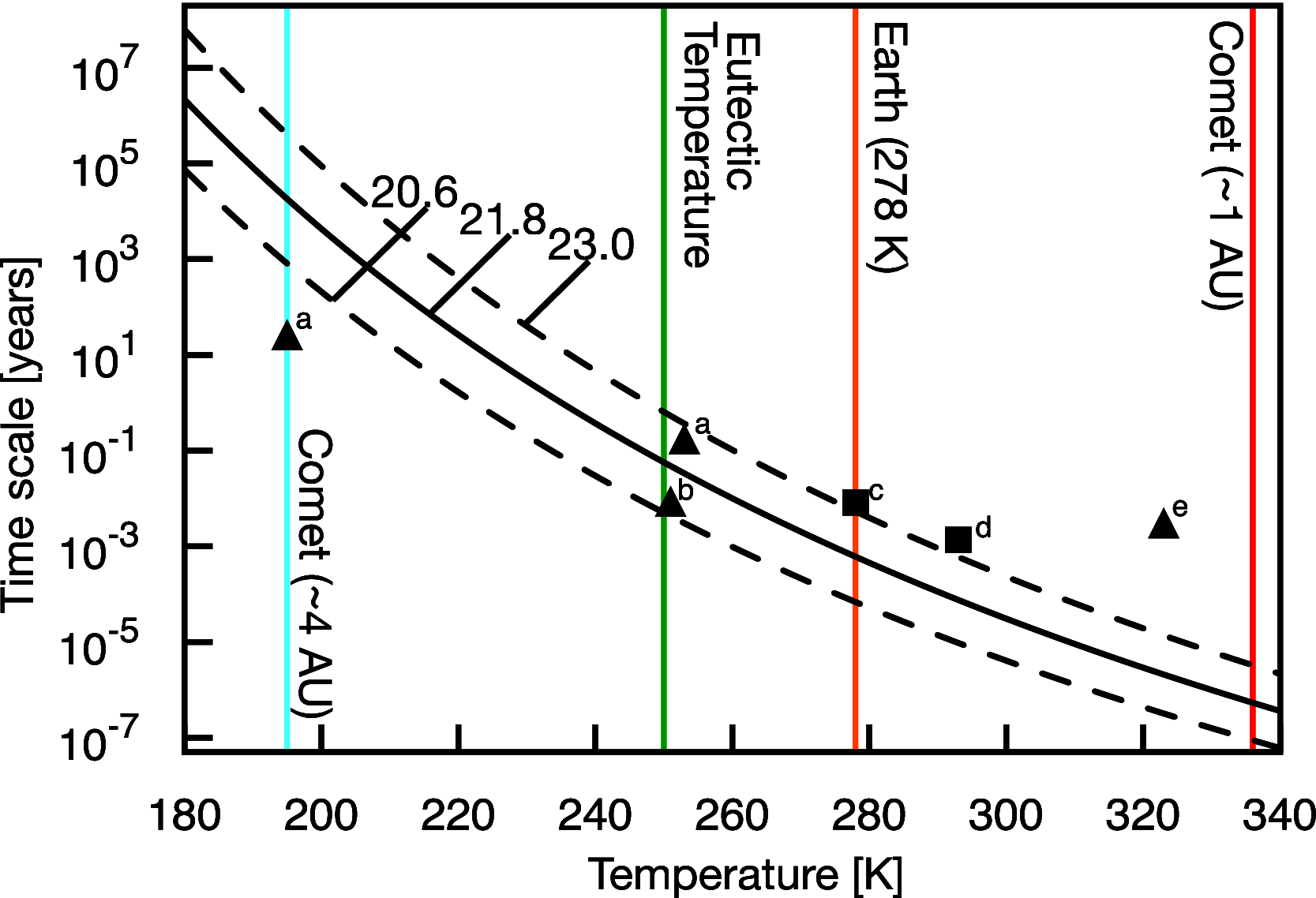
Hydrogen cyanide (HCN) is known to react with complex organic materials and is a key reagent in the formation of various prebiotic building blocks, including amino acids and nucleobases. Here, we explore the possible first step in several such processes, the dimerization of HCN into iminoacetonitrile. Our study combines steered ab initio molecular dynamics and quantum chemistry to evaluate the kinetics and thermodynamics of base-catalyzed dimerization of HCN in the liquid state. Simulations predict a formation mechanism of iminoacetonitrile that is consistent with experimentally observed time scales for HCN polymerization, suggesting that HCN dimerization may be the rate-determining step in the assembly of more complex reaction products. The predicted kinetics permits for iminoacetonitrile formation in a host of astrochemical environments, including on the early Earth, on periodically heated subsurfaces of comets, and following heating events on colder bodies, such as Saturn's moon Titan.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
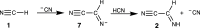

References
LinkOut - more resources
Full Text Sources
