A Randomized Trial of Calcium Plus Vitamin D Supplementation and Risk of Ductal Carcinoma In Situ of the Breast
- PMID: 34476342
- PMCID: PMC8406436
- DOI: 10.1093/jncics/pkab072
A Randomized Trial of Calcium Plus Vitamin D Supplementation and Risk of Ductal Carcinoma In Situ of the Breast
Abstract
Background: The effect of calcium plus vitamin D (CaD) supplementation on risk of ductal carcinoma in situ (DCIS) of the breast, a nonobligate precursor of invasive ductal carcinoma, is not well understood. In this secondary analysis, we examined this association in the Women's Health Initiative CaD trial over approximately 20 years of follow-up.
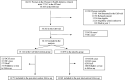
Methods: A total of 36 282 cancer-free postmenopausal women (50-79 years) were randomly assigned to daily (d) calcium (1000 mg) plus vitamin D (400 IU) supplementation or to a placebo. Personal supplementation with vitamin D (≤600 IU/d, subsequently raised to 1000 IU/d) and calcium (≤1000 mg/d) was allowed. The intervention phase (median = 7.1 years), was followed by a postintervention phase (additional 13.8 years), which included 86.0% of the surviving women. A total of 595 incident DCIS cases were ascertained. Hazard ratios (HRs) plus 95% confidence intervals (CIs) were calculated.
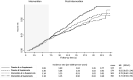
Results: The intervention group had a lower risk of DCIS throughout follow-up (HR = 0.82, 95% CI = 0.70 to 0.96) and during the postintervention phase (HR = 0.76, 95% CI = 0.61 to 0.94). The group that used CaD personal supplements in combination with the trial intervention had a lower risk of DCIS compared with the trial placebo group that did not use personal supplementation (HR = 0.72, 95% CI = 0.56 to 0.91).
Conclusions: CaD supplementation in postmenopausal women was associated with reduced risk of DCIS, raising the possibility that consistent use of these supplements might provide long-term benefits for the prevention of DCIS.
© The Author(s) 2021. Published by Oxford University Press.
Figures


References
-
- Allegra CJ, Aberle DR, Ganschow P, et al.National Institutes of Health state-of-the-science conference statement: diagnosis and management of ductal carcinoma in situ September 22-24, 2009. J Natl Cancer Inst. 2010;102(3):161–169. - PubMed
-
- Erbas B, Provenzano E, Armes J, Gertig D.. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat. 2006;97(2):135–144. - PubMed
-
- Page DL, Dupont WD, Rogers LW, Jensen RA, Schuyler PA.. Continued local recurrence of carcinoma 15-25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer. 1995;76(7):1197–1200. - PubMed
-
- Wohlfahrt J, Rank F, Kroman N, Melbye M.. A comparison of reproductive risk factors for CIS lesions and invasive breast cancer. Int J Cancer. 2004;108(5):750–753. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
