Biophysical Investigation of Sodium Channel Interaction with β-Subunit Variants Associated with Arrhythmias
- PMID: 34476357
- PMCID: PMC8370339
- DOI: 10.1089/bioe.2020.0030
Biophysical Investigation of Sodium Channel Interaction with β-Subunit Variants Associated with Arrhythmias
Abstract
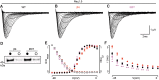
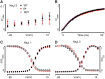
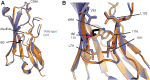
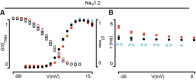
Background: Voltage-gated sodium (NaV) channels help regulate electrical activity of the plasma membrane. Mutations in associated subunits can result in pathological outcomes. Here we examined the interaction of NaV channels with cardiac arrhythmia-linked mutations in SCN2B and SCN4B, two genes that encode auxiliary β-subunits. Materials and Methods: To investigate changes in SCN2B R137H and SCN4B I80T function, we combined three-dimensional X-ray crystallography with electrophysiological measurements on NaV1.5, the dominant subtype in the heart. Results: SCN4B I80T alters channel activity, whereas SCN2B R137H does not have an apparent effect. Structurally, the SCN4B I80T perturbation alters hydrophobic packing of the subunit with major structural changes and causes a thermal destabilization of the folding. In contrast, SCN2B R137H leads to structural changes but overall protein stability is unaffected. Conclusion: SCN4B I80T data suggest a functionally important region in the interaction between NaV1.5 and β4 that, when disrupted, could lead to channel dysfunction. A lack of apparent functional effects of SCN2B R137H on NaV1.5 suggests an alternative working mechanism, possibly through other NaV channel subtypes present in heart tissue. Indeed, mapping the structural variations of SCN2B R137H onto neuronal NaV channel structures suggests altered interaction patterns.
Keywords: NaV1.5; SCN2B; SCN4B; arrhythmia.
Copyright 2020, Mary Ann Liebert, Inc., publishers.
Conflict of interest statement
No competing financial interests exist.
Figures




References
-
- Rook MB, Evers MM, Vos MA, et al. . Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc Res 2012;93:12–23 - PubMed
-
- Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol 1992;20:1391–1396 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
