Exercise performance in patients with post-acute sequelae of SARS-CoV-2 infection compared to patients with unexplained dyspnea
- PMID: 34476393
- PMCID: PMC8401400
- DOI: 10.1016/j.eclinm.2021.101066
Exercise performance in patients with post-acute sequelae of SARS-CoV-2 infection compared to patients with unexplained dyspnea
Abstract
Background: Dyspnea and exercise intolerance are commonly reported post-acute sequelae of SARS-CoV-2 infection (PASC), but routine diagnostic testing is often normal. Cardiopulmonary exercise testing (CPET) offers comprehensive assessment of dyspnea to characterize pulmonary PASC.
Methods: We performed a retrospective cohort study of CPET performed on patients reporting dyspnea and/or exercise intolerance following confirmed Covid-19 between August 1, 2020 and March 1, 2021, and compared them to age- and sex-matched patients with unexplained dyspnea referred for CPET at the same center in the pre-Covid-19 era.
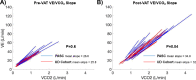
Findings: Compared to matched unexplained dyspnea comparators, PASC patients shared similar medical comorbidities and subjective dyspnea at referral (mMRC score 1.6 ± 0.9 vs. 1.4 ± 0.9, P = 0.5). Fifteen (83.3%) PASC patients underwent high resolution computed tomography of the chest, of which half (46.7%) were normal, and 17 (94.4%) patients had pulmonary function testing, of which the majority (76.5%) were normal. All patients underwent CPET, and 12 (67%) had normal findings. Compared to matched comparators, PASC patients had similar peak oxygen consumption, oxygen consumption at ventilatory anaerobic threshold, and ventilatory efficiency measured by the minute ventilation to carbon dioxide production (VE/VCO2) slope.
Interpretation: Despite prominent dyspnea, physiological abnormalities on CPET were mild across a range of initial Covid-19 severity and similar to matched comparators referred for dyspnea without antecedent SARS-CoV-2.
Funding: The project was supported by the NHLBI (R01HL131029, R01HL151841, U10HL110337, T32HL116275) and a KL2 award (5KL2TR002542-02) from Harvard Catalyst.
Keywords: COVID-19; Cardiopulmonary exercise test; Dyspnea; Exercise intolerance; Post-acute sequelae of SARS-CoV-2 infection; SARS-CoV-2.
© 2021 The Authors.
Conflict of interest statement
LPH receives consulting fees from Plaint Therapeutics and Boehringer Ingelheim and serves on the medical advisory board Boehringer Ingelheim, all unrelated to the content of this manuscript. BDM is a data safety monitoring board member for a clinical trial (Multicenter, Randomized, Double-Blinded, Placebo-Controlled Study to Assess Safety and Efficacy of SIR1–365 in Patients with Severe COVID-19) funded by Sironax, USA, unrelated to the content of this manuscript; received payment from Abbvie for providing a presentation on severe COVID-19. GDL serves on the steering committee for clinical trials related to CPET for Amgen, AstraZeneca, Cyclerion, and Cytokinetics, all unrelated to the content of this manuscript; and Cardiopulmonary Exercise Testing Core Laboratory Projects with NHLBI, Amgen, AstraZeneca, Cyclerion, Cytokinetics, Applied Therapeutics, and Abbott, that are not directly related to this work.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

