Diagnostic performance of a colorimetric RT -LAMP for the identification of SARS-CoV-2: A multicenter prospective clinical evaluation in sub-Saharan Africa
- PMID: 34476394
- PMCID: PMC8401528
- DOI: 10.1016/j.eclinm.2021.101101
Diagnostic performance of a colorimetric RT -LAMP for the identification of SARS-CoV-2: A multicenter prospective clinical evaluation in sub-Saharan Africa
Abstract
Background: Management and control of the COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus SARS-CoV-2 is critically dependent on quick and reliable identification of the virus in clinical specimens. Detection of viral RNA by a colorimetric reverse transcription loop-mediated isothermal amplification (RT-LAMP) is a simple, reliable and cost-effective assay, deployable in resource-limited settings (RLS). Our objective was to evaluate the intrinsic and extrinsic performances of RT-LAMP in RLS.
Methods: This is a multicenter prospective observational study of diagnostic accuracy, conducted from October 2020 to February 2021 in four African Countries: Cameroon, Ethiopia, Kenya and Nigeria; and in Italy. We enroled 1657 individuals who were either COVID-19 suspect cases, or asymptomatic and presented for screening. RNA extracted from pharyngeal swabs was tested in parallel by a colorimetric RT-LAMP and by a standard real time polymerase chain reaction (RT-PCR).
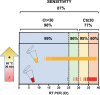
Findings: The sensitivity and specificity of index RT LAMP compared to standard RT-PCR on 1657 prospective specimens from infected individuals was determined. For a subset of 1292 specimens, which underwent exactly the same procedures in different countries, we obtained very high specificity (98%) and positive predictive value (PPV = 99%), while the sensitivity was 87%, with a negative predictive value NPV = 70%, Stratification of RT-PCR data showed superior sensitivity achieved with an RT-PCR cycle threshold (Ct) below 35 (97%), which decreased to 60% above 35.
Interpretation: In this field trial, RT-LAMP appears to be a reliable assay, comparable to RT-PCR, particularly with medium-high viral loads (Ct < 35). Hence, RT-LAMP can be deployed in RLS for timely management and prevention of COVID-19, without compromising the quality of output.
© 2021 The Authors.
Conflict of interest statement
The authors declare that this study was supported by the Bill & Melinda Gates Foundation [Grant Number INV-022,816]. Furthermore, New England Biolabs provided reagents free of charge. The authors have no additional interests to declare.
Figures
References
-
- WHO: diagnostic testing for SARS-CoV-2 - interim guidance, 2020.
-
- WHO. Increasing access to diagnostics through technology transfer and local production. 2011. https://www.who.int/publications/i/item/9789241502375 (accessed April 2021).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

