Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination in a high-risk national population
- PMID: 34476395
- PMCID: PMC8400515
- DOI: 10.1016/j.eclinm.2021.101117
Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination in a high-risk national population
Abstract
Background: Breakthrough infections after SARS-CoV-2 infection have been reported. Clinical outcomes among persons with breakthrough infection are not known.
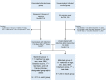
Methods: We retrospectively identified all Veterans with a confirmed SARS-CoV-2 infection >14 days after the second dose of either Pfizer-BNT-162b2 or Moderna-mRNA-1273 vaccine between December 15, 2020 and March 30, 2021, and age, race, sex, body mass index, Charlson comorbidity index, geographical location, and date of positive test matched unvaccinated controls with SARS-CoV-2 infection. Our primary endpoint was the rate or severe disease defined as hospitalization, mechanical ventilation, or death in both groups.
Findings: Among 258,716 persons with both doses of vaccines and 756,150 without any vaccination, we identified 271 (0.1%) vaccinated persons with breakthrough infection and 48,114 (6.4%) unvaccinated matched controls with infection between December 15, 2020 and March 30, 2021. Among 213 matched pairs, symptoms were present in 33.3% of those with breakthrough infection and 42.2% of the controls. A total of 79 persons met the definition of severe disease or death (42 in the breakthrough infection group and 37 in the control group). Rate of severe disease or death per 1,000 person-days (95% CI) was 4.08 (2.64,5.31) among those with breakthrough infection and 3.6 (2.53,4.73) among the controls (P = 0.58). Rate was similar among both groups regardless of age-group, race, BMI or presence of comorbidities. Among persons with breakthrough infection and matched controls with infection, vaccination was not associated with a lower risk of severe disease or death in the main analyses but was associated with a lower risk when matching did not include geographic location (HR 0.62, 95% CI 0.43,0.91).
Interpretation: Demographic or clinical factors are not associated with a lower risk of severe disease or death in persons with breakthrough SARS-CoV-2 infection.
Funding: None.
Keywords: Breakthrough infection; Outcomes; Sars-CoV-2; Vaccination.
© 2021 The Authors.
Conflict of interest statement
Dr. Butt has received grants (to the institution) from Gilead Sciences. Dr. Mayr is supported by K23GM132688 from the National Institutes of Health. Other authors have no relevant disclosures.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

