Biomarker-Based Classification of Patients With Acute Respiratory Failure Into Inflammatory Subphenotypes: A Single-Center Exploratory Study
- PMID: 34476405
- PMCID: PMC8378789
- DOI: 10.1097/CCE.0000000000000518
Biomarker-Based Classification of Patients With Acute Respiratory Failure Into Inflammatory Subphenotypes: A Single-Center Exploratory Study
Abstract
Objectives: Hyper- and hypoinflammatory subphenotypes discovered in patients with acute respiratory distress syndrome predict clinical outcomes and therapeutic responses. These subphenotypes may be important in broader critically ill patient populations with acute respiratory failure regardless of clinical diagnosis. We investigated subphenotyping with latent class analysis in an inclusive population of acute respiratory failure, derived a parsimonious model for subphenotypic predictions based on a small set of variables, and examined associations with clinical outcomes.
Design: Prospective, observational cohort study.
Setting: Single-center, academic medical ICU.
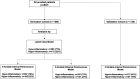
Patients: Mechanically ventilated patients with acute respiratory failure.
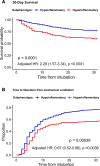
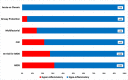
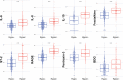
Measurements and main results: We included 498 patients with acute respiratory failure (acute respiratory distress syndrome: 143, at-risk for acute respiratory distress syndrome: 198, congestive heart failure: 37, acute on chronic respiratory failure: 23, airway protection: 61, and multifactorial: 35) in our derivation cohort and measured 10 baseline plasma biomarkers. Latent class analysis considering clinical variables and biomarkers determined that a two-class model offered optimal fit (23% hyperinflammatory subphenotype). Distribution of hyperinflammatory subphenotype varied among acute respiratory failure etiologies (acute respiratory distress syndrome: 31%, at-risk for acute respiratory distress syndrome: 27%, congestive heart failure: 22%, acute on chronic respiratory failure 0%, airway protection: 5%, and multifactorial: 14%). Hyperinflammatory patients had higher Sequential Organ Failure Assessment scores, fewer ventilator-free days, and higher 30- and 90-day mortality (all p < 0.001). We derived a parsimonious model consisting of angiopoietin-2, soluble tumor necrosis factor receptor-1, procalcitonin, and bicarbonate and classified subphenotypes in a validation cohort (n = 139). Hyperinflammatory patients (19%) demonstrated higher levels of inflammatory biomarkers not included in the model (p < 0.01) and worse outcomes.
Conclusions: Host-response subphenotypes are observable in a heterogeneous population with acute respiratory failure and predict clinical outcomes. Simple, biomarker-based models can offer prognostic enrichment in patients with acute respiratory failure. The differential distribution of subphenotypes by specific etiologies of acute respiratory failure indicates that subphenotyping may be more relevant in patients with hypoxemic causes of acute respiratory failure and not in patients intubated for airway protection or acute on chronic decompensation.
Keywords: acute respiratory distress syndrome; acute respiratory failure; biomarkers; heterogeneity; latent class analysis; subphenotypes.
Copyright © 2021 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
Dr. McVerry has been a consultant for Boehringer-Ingelheim, Inc. and receives research funding from Bayer Pharmaceuticals, Inc. Dr. Kitsios has received research funding from Karius, Inc. The remaining authors have disclosed that they do not have any conflicts of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

