A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida)
- PMID: 34476645
- PMCID: PMC8558154
- DOI: 10.1007/s10549-021-06367-5
A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida)
Abstract
Purpose: CXCR1, one of the receptors for CXCL8, has been identified as a druggable target on breast cancer cancer stem cells (CSC). Reparixin (R), an investigational oral inhibitor of CXCR1, was safely administered to metastatic breast cancer patients in combination with paclitaxel (P) and appeared to reduce CSC in a window-of-opportunity trial in operable breast cancer. The fRida trial (NCT02370238) evaluated the addition of R to weekly as first-line therapy for metastatic (m) TNBC.
Subjects and methods: Subjects with untreated mTNBC were randomized 1:1 to R or placebo days 1-21 in combination with weekly P 80 mg/m2 on days 1, 8, 15 of 28-day cycles. The primary endpoint was PFS by central review.
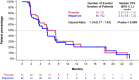
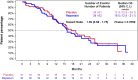
Results: 123 subjects were randomized (62 to R + P and 61 to placebo + P). PFS was not different between the 2 groups (median 5.5 and 5.6 months for R + P and placebo + P, respectively; HR 1.13, p = 0.5996). ALDH+ and CD24-/CD44+ CSC centrally evaluated by IHC were found in 16 and 34 of the 54 subjects who provided a metastatic tissue biopsy at study entry. Serious adverse events (21.3 and 20% of subjects) and grade ≥ 3 adverse reactions (ADR) (9.1 and 6.3% of all ADRs) occurred at similar frequency in both groups.
Conclusion: fRida is the first randomized, double-blind clinical trial of a CSC-targeting agent in combination with chemotherapy in breast cancer. The primary endpoint of prolonged PFS was not met.
Clinical trial registration/date of registration: NCT01861054/February 24, 2015.
Keywords: CXCR1; Cancer stem cells; Reparixin; TNBC.
© 2021. The Author(s).
Conflict of interest statement
PAR, SMC, and BB are full-time employees of Dompé farmaceutici s.p.a. All other authors declare that they have no competing interests.
Figures
References
-
- Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Can Res. 2009;69(4):1302–1313. doi: 10.1158/0008-5472.can-08-2741. - DOI - PMC - PubMed
-
- Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Investig. 2010;120(2):485–497. doi: 10.1172/jci39397. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

