Leveraging Case Narratives to Enhance Patient Age Ascertainment from Adverse Event Reports
- PMID: 34476768
- PMCID: PMC9136956
- DOI: 10.1007/s40290-021-00398-5
Leveraging Case Narratives to Enhance Patient Age Ascertainment from Adverse Event Reports
Abstract
Introduction: Missing age presents a significant challenge when evaluating individual case safety reports (ICSRs) in the FDA Adverse Event Reporting System (FAERS). When age is missing in an ICSR's structured field, it may be in the report's free-text narrative.
Objectives: This study aimed to evaluate the performance and assess the potential impact of a rule-based natural language processing (NLP) tool that utilizes a text string search to identify patients' numerical age from unstructured narratives.
Methods: Using FAERS ICSRs from 2002 to 2018, we evaluated the annual proportion of ICSRs with age missing in the structured field before and after NLP application. Reviewers manually identified patients' age from ICSR narratives (gold standard) from a random sample of 1500 ICSRs. The gold standard was compared to the NLP-identified age.
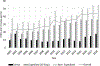
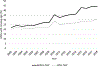
Results: During the study period, the percentage of ICSRs missing age in the structured field increased from 21.9 to 43.8%. The NLP tool performed well among the random sample: sensitivity 98.5%, specificity 92.9%, positive predictive value (PPV) 94.9%, and F-measure 96.7%. It also performed well for the subset of ICSRs missing age in the structured field; when applied to these cases, NLP identified age for an additional one million ICSRs (10% of the total number of ICSRs from 2002 to 2018) and decreased the percentage of ICSRs missing age to 27% overall.
Conclusions: NLP has potential utility to extract patients' age from ICSR narratives. Use of this tool would enhance pharmacovigilance and research using FAERS data.
© 2021. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
Declarations
Figures
Similar articles
-
An Evaluation of Postmarketing Reports with an Outcome of Death in the US FDA Adverse Event Reporting System.Drug Saf. 2020 May;43(5):457-465. doi: 10.1007/s40264-020-00908-5. Drug Saf. 2020. PMID: 31981082
-
Towards Automating Adverse Event Review: A Prediction Model for Case Report Utility.Drug Saf. 2020 Apr;43(4):329-338. doi: 10.1007/s40264-019-00897-0. Drug Saf. 2020. PMID: 31912439
-
Evaluation of Natural Language Processing (NLP) systems to annotate drug product labeling with MedDRA terminology.J Biomed Inform. 2018 Jul;83:73-86. doi: 10.1016/j.jbi.2018.05.019. Epub 2018 Jun 1. J Biomed Inform. 2018. PMID: 29860093
-
Development of a multivariate prediction model to identify individual case safety reports which require clinical review.Pharmacoepidemiol Drug Saf. 2022 Dec;31(12):1300-1307. doi: 10.1002/pds.5553. Epub 2022 Oct 21. Pharmacoepidemiol Drug Saf. 2022. PMID: 36251280 Review.
-
Leveraging Natural Language Processing and Machine Learning Methods for Adverse Drug Event Detection in Electronic Health/Medical Records: A Scoping Review.Drug Saf. 2025 Apr;48(4):321-337. doi: 10.1007/s40264-024-01505-6. Epub 2025 Jan 9. Drug Saf. 2025. PMID: 39786481 Free PMC article.
Cited by
-
Detection Algorithms for Simple Two-Group Comparisons Using Spontaneous Reporting Systems.Drug Saf. 2024 Jun;47(6):535-543. doi: 10.1007/s40264-024-01404-w. Epub 2024 Feb 22. Drug Saf. 2024. PMID: 38388828 Review.
-
Challenges and opportunities for mining adverse drug reactions: perspectives from pharma, regulatory agencies, healthcare providers and consumers.Database (Oxford). 2022 Sep 2;2022:baac071. doi: 10.1093/database/baac071. Database (Oxford). 2022. PMID: 36050787 Free PMC article.
References
-
- US Food and Drug Administration. Questions and answers on FDA’s Adverse Event Reporting System (FAERS) https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-advers.... Accessed 25 Nov 2019
-
- De Salas CSRL. Pharmacovigilance in pediatric population. In: Pharmacovigilance IntechOpen; 2019.
-
- Moore TJ, Furberg CD, Mattison DR, Cohen MR. Completeness of serious adverse drug event reports received by the US Food and Drug Administration in 2014. Pharmacoepidemiol Drug Saf 2016;25(6):713–8. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

