Investigating the Barrier Activity of Novel, Human Enhancer-Blocking Chromatin Insulators for Hematopoietic Stem Cell Gene Therapy
- PMID: 34477013
- PMCID: PMC9206477
- DOI: 10.1089/hum.2021.142
Investigating the Barrier Activity of Novel, Human Enhancer-Blocking Chromatin Insulators for Hematopoietic Stem Cell Gene Therapy
Abstract
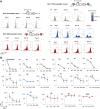
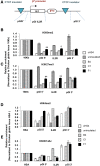
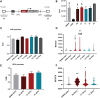
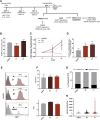
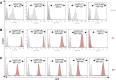
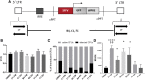
Despite the unequivocal success of hematopoietic stem and progenitor cell gene therapy, limitations still exist including genotoxicity and variegation/silencing of transgene expression. A class of DNA regulatory elements known as chromatin insulators (CIs) can mitigate both vector transcriptional silencing (barrier CIs) and vector-induced genotoxicity (enhancer-blocking CIs) and have been proposed as genetic modulators to minimize unwanted vector/genome interactions. Recently, a number of human, small-sized, and compact CIs bearing strong enhancer-blocking activity were identified. To ultimately uncover an ideal CI with a dual, enhancer-blocking and barrier activity, we interrogated these elements in vitro and in vivo. After initial screening of a series of these enhancer-blocking insulators for potential barrier activity, we identified three distinct categories with no, partial, or full protection against transgene silencing. Subsequently, the two CIs with full barrier activity (B4 and C1) were tested for their ability to protect against position effects in primary cells, after incorporation into lentiviral vectors (LVs) and transduction of human CD34+ cells. B4 and C1 did not adversely affect vector titers due to their small size, while they performed as strong barrier insulators in CD34+ cells, both in vitro and in vivo, shielding transgene's long-term expression, more robustly when placed in the forward orientation. Overall, the incorporation of these dual-functioning elements into therapeutic viral vectors will potentially provide a new generation of safer and more efficient LVs for all hematopoietic stem cell gene therapy applications.
Keywords: CTCF-binding site; barrier activity; chromatin insulator; gene therapy; hematopoietic stem cells.
Conflict of interest statement
The authors declare that they have no competing interests relevant to the subject matter of this article.
Figures

Similar articles
-
Identification and characterization of enhancer-blocking insulators to reduce retroviral vector genotoxicity.PLoS One. 2013 Oct 3;8(10):e76528. doi: 10.1371/journal.pone.0076528. eCollection 2013. PLoS One. 2013. PMID: 24098520 Free PMC article.
-
Genomic discovery of potent chromatin insulators for human gene therapy.Nat Biotechnol. 2015 Feb;33(2):198-203. doi: 10.1038/nbt.3062. Epub 2015 Jan 12. Nat Biotechnol. 2015. PMID: 25580597
-
Chicken HS4 insulators have minimal barrier function among progeny of human hematopoietic cells transduced with an HIV1-based lentiviral vector.Mol Ther. 2011 Jan;19(1):133-9. doi: 10.1038/mt.2010.218. Epub 2010 Oct 12. Mol Ther. 2011. PMID: 20940706 Free PMC article.
-
The use of chromatin insulators to improve the expression and safety of integrating gene transfer vectors.Hum Gene Ther. 2011 Jun;22(6):761-74. doi: 10.1089/hum.2010.233. Epub 2011 Mar 25. Hum Gene Ther. 2011. PMID: 21247248 Free PMC article. Review.
-
Stem cell gene therapy, position effects and chromatin insulators.Stem Cells. 1997;15 Suppl 1:265-71. doi: 10.1002/stem.5530150834. Stem Cells. 1997. PMID: 9368350 Review.
Cited by
-
Large-scale discovery of potent, compact and erythroid specific enhancers for gene therapy vectors.Nat Commun. 2025 May 9;16(1):4325. doi: 10.1038/s41467-025-59235-x. Nat Commun. 2025. PMID: 40346084 Free PMC article.
-
Promises and Pitfalls of Next-Generation Treg Adoptive Immunotherapy.Cancers (Basel). 2023 Dec 17;15(24):5877. doi: 10.3390/cancers15245877. Cancers (Basel). 2023. PMID: 38136421 Free PMC article. Review.
-
Epigenetic Regulation of Erythropoiesis: From Developmental Programs to Therapeutic Targets.Int J Mol Sci. 2025 Jun 30;26(13):6342. doi: 10.3390/ijms26136342. Int J Mol Sci. 2025. PMID: 40650116 Free PMC article. Review.
References
-
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. . Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000;288:669–672. - PubMed
-
- Wu X, Li Y, Crise B, et al. . Transcription start regions in the human genome are favored targets for MLV integration. Science 2003;300:1749–1751. - PubMed
-
- Schröder ARW, Shinn P, Chen H, et al. . HIV-1 integration in the human genome favors active genes and local hotspots. Cell 2002;110:521–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

