Simple Measurement of IgA Predicts Immunity and Mortality in Ataxia-Telangiectasia
- PMID: 34477998
- PMCID: PMC8604875
- DOI: 10.1007/s10875-021-01090-8
Simple Measurement of IgA Predicts Immunity and Mortality in Ataxia-Telangiectasia
Abstract
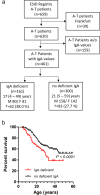
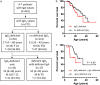
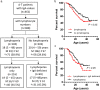
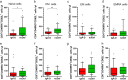
Patients with ataxia-telangiectasia (A-T) suffer from progressive cerebellar ataxia, immunodeficiency, respiratory failure, and cancer susceptibility. From a clinical point of view, A-T patients with IgA deficiency show more symptoms and may have a poorer prognosis. In this study, we analyzed mortality and immunity data of 659 A-T patients with regard to IgA deficiency collected from the European Society for Immunodeficiencies (ESID) registry and from 66 patients with classical A-T who attended at the Frankfurt Goethe-University between 2012 and 2018. We studied peripheral B- and T-cell subsets and T-cell repertoire of the Frankfurt cohort and survival rates of all A-T patients in the ESID registry. Patients with A-T have significant alterations in their lymphocyte phenotypes. All subsets (CD3, CD4, CD8, CD19, CD4/CD45RA, and CD8/CD45RA) were significantly diminished compared to standard values. Patients with IgA deficiency (n = 35) had significantly lower lymphocyte counts compared to A-T patients without IgA deficiency (n = 31) due to a further decrease of naïve CD4 T-cells, central memory CD4 cells, and regulatory T-cells. Although both patient groups showed affected TCR-ß repertoires compared to controls, no differences could be detected between patients with and without IgA deficiency. Overall survival of patients with IgA deficiency was significantly diminished. For the first time, our data show that patients with IgA deficiency have significantly lower lymphocyte counts and subsets, which are accompanied with reduced survival, compared to A-T patients without IgA deficiency. IgA, a simple surrogate marker, is indicating the poorest prognosis for classical A-T patients. Both non-interventional clinical trials were registered at clinicaltrials.gov 2012 (Susceptibility to infections in ataxia-telangiectasia; NCT02345135) and 2017 (Susceptibility to Infections, tumor risk and liver disease in patients with ataxia-telangiectasia; NCT03357978).
Keywords: Ataxia-telangiectasia; IgA deficiency; Immunodeficiency; Immunoglobulins; Lymphopenia; Mortality.
© 2021. The Author(s).
Conflict of interest statement
The authors have the following conflicts to declare:
Dr. Zielen reports honoraria from CSL-Behring, AstraZeneca; Sanofi-Aventis GmbH; Boehringer Ingelheim; Stallergenes Greer; Allergy Therapeutics, bene-Arzneimittel GmbH; Vifor Pharma GmbH; Novartis AG; GlaxoSmithKline GmbH; ALK-Abelló Arzneimittel GmbH; Lofarma GmbH, IMS Health GmbH & Co; and Biotest Pharma GmbH, outside the submitted work.
Dr. Ehl reports grants from UCB, during the conduct of the study.
Dr. Sabine M. El-Helou was funded by the Federal Ministry of Education and Research (BMBF), by the European Society for Immunodeficiencies (ESID), by the Care-for-Rare Foundation, by PROimmun e.V., and by a restricted grant from LFB, CSL Behring, and Grifols. For GAIN and RESIST, she was funded by the BMBF.
Dr. Kindle reports grants from ESID (European Society for Immunodeficiencies) and grants from BMBF (German Federal Ministry of Education and Research), during the conduct of the study.
Dr. van Aerde reports grants from Baxter, during the conduct of the study.
Dr. Laws reports personal fees from Shire; Roche; Novonordisk; Pfizer; Csl Behring, outside the submitted work.
Dr. Niehues reports honoraria from uptodate.com; travelling costs from PENTA and JIR cohorts (Padua, Italy; Lausanne Switzerland), up to 2017 from PPTA (plasma protein therapeutics association).
Dr. Seidel reports personal fees from Shire [Hypogammaglobulinemia (Subcutaneous IgG, hyaluronic acid, immune globulin (human))], outside the submitted work.
Dr. Meyts reports grants from CSL Behring, outside the submitted work. IM is a senior clinical investigator at FWO Vlaanderen (supported by CSL Behring Chair of Primary Immunodeficiencies, by a KU Leuven C1 Grant C16/18/007, by a VIB GC PID Grant, by a FWO Grants G0C8517N; and G0E8420N and by the Jeffrey Modell Foundation. IM is a recipient of a ERC-StG MORE2ADA2. This work is supported by ERN-RITA.
Dr. Grimbacher reports grants from DZIF, DFG, BMBF, Fritz Thyssen Stiftung, Shire/Baxalta, Merck, BMS, Novartis, CSL Behring, personal fees from Janssen Cilag, Novartis, during the conduct of the study, outside the submitted work.
Dr. Schubert reports grants from A-T Children’s Project, DFG, SPARKS–Action for A-T, Starke Lunge Foundation, personal fees from Biotest Pharma GmbH and Vifor Pharma Deutschland GmbH, outside the submitted work.
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Boder E, Sedgwick RP. Ataxia-telangiectasia; a familial syndrome of progressive cerebellar ataxia, oculocutaneous telangiectasia and frequent pulmonary infection. Pediatrics. 1958;21:526–554. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

