Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-β upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury
- PMID: 34478241
- PMCID: PMC8408371
- DOI: 10.1002/jev2.12137
Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-β upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury
Abstract
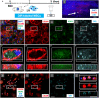
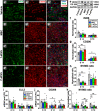
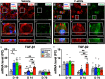
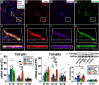
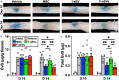
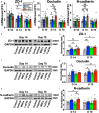
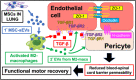
Intravenous (IV) infusion of bone marrow-derived mesenchymal stem/stromal cells (MSCs) stabilizes the blood-spinal cord barrier (BSCB) and improves functional recovery in experimental models of spinal cord injury (SCI). Although IV delivered MSCs do not traffic to the injury site, IV delivered small extracellular vesicles (sEVs) derived from MSCs (MSC-sEVs) do and are taken up by a subset of M2 macrophages. To test whether sEVs released by MSCs are responsible for the therapeutic effects of MSCs, we tracked sEVs produced by IV delivered DiR-labelled MSCs (DiR-MSCs) after transplantation into SCI rats. We found that sEVs were released by MSCs in vivo, trafficked to the injury site, associated specifically with M2 macrophages and co-localized with exosome markers. Furthermore, while a single MSC injection was sufficient to improve locomotor recovery, fractionated dosing of MSC-sEVs over 3 days (F-sEVs) was required to achieve similar therapeutic effects. Infusion of F-sEVs mimicked the effects of single dose MSC infusion on multiple parameters including: increased expression of M2 macrophage markers, upregulation of transforming growth factor-beta (TGF-β), TGF-β receptors and tight junction proteins, and reduction in BSCB permeability. These data suggest that release of sEVs by MSCs over time induces a cascade of cellular responses leading to improved functional recovery.
Keywords: blood-spinal cord barrier; exosomes; macrophages; mesenchymal stem/stromal cells; small extracellular vesicles; spinal cord injury; transforming growth factor-beta.
© 2021 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- Basso, D. M., Beattie, M. S., & Bresnahan, J. C. (1996). Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight‐drop device versus transection. Experimental Neurology, 139:244–256. - PubMed
-
- Beck, K. D., Nguyen, H. X., Galvan, M. D., Salazar, D. L., Woodruff, T. M., & Anderson, A. J. (2010). Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain, 133, 433–447. - PMC - PubMed
-
- Bradbury, E. J., Moon, L. D., Popat, R. J., King, V. R., Bennett, G. S., Patel, P. N., Fawcett, J. W., & McMahon, S. B. (2002). Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature, 416, 636–640. - PubMed
-
- Chen, H. J., Li Yim, A. Y. F., Griffith, G. R., de Jonge, W. J., Mannens, M., Ferrero, E., Henneman, P., & de Winther, M. P. J. (2019). Meta‐analysis of in vitro‐differentiated macrophages identifies transcriptomic signatures that classify disease macrophages in vivo. Frontiers in Immunology, 10, 2887. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

