Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma
- PMID: 34478487
- PMCID: PMC8945634
- DOI: 10.1182/bloodadvances.2020003848
Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma
Abstract
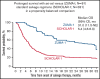
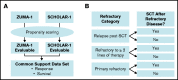
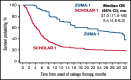
The SCHOLAR-1 international retrospective study highlighted poor clinical outcomes and survival among patients with refractory large B-cell lymphoma (LBCL) treated with conventional chemotherapy. Axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, demonstrated durable responses in patients with refractory LBCL in the pivotal phase 1/2 ZUMA-1 study (NCT02348216). Here, we compared SCHOLAR-1 with the 2-year outcomes of ZUMA-1. Prior to comparison of clinical outcomes, propensity scoring (based on a broad set of prognostic covariates) was used to create balance between ZUMA-1 and SCHOLAR-1 patients. In the pivotal phase 2 portion of ZUMA-1, 101 patients received axi-cel and were evaluable for response and survival. In SCHOLAR-1, 434 and 424 patients were evaluable for response and survival, respectively. ZUMA-1 patients were more heavily pretreated than were SCHOLAR-1 patients. The median follow-up was 27.1 months in ZUMA-1. The objective response rate (ORR) and complete response rate were 83% and 54% in ZUMA-1 vs 34% and 12% in SCHOLAR-1, respectively. The 2-year survival rate was 54% in ZUMA-1 and 20% in SCHOLAR-1, and a 73% reduction in the risk of death was observed in ZUMA-1 vs SCHOLAR-1. These results were consistent with those of an additional standardization analysis in which strata were limited to 2 prognostic factors (refractory categorization and presence/absence of stem cell transplant after refractoriness to chemotherapy) to conserve sample size. Despite the limitations of a nonrandomized analysis, these results indicate that axi-cel produces durable responses and a substantial survival benefit vs non-CAR T-cell salvage regimens for patients with refractory LBCL.
© 2021 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures
References
-
- Surveillance, Epidemiology and End Results Program. SEER Cancer Statistics Review, 1975-2013. Available at: https://seer.cancer.gov/archive/csr/1975_2013/. Accessed 4 May 2021.
-
- Sant M, Minicozzi P, Mounier M, et al. ; EUROCARE-5 Working Group . Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol. 2014;15(9):931-942. - PubMed
-
- Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR.. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459. - PubMed

