Outcomes of patients with CLL sequentially resistant to both BCL2 and BTK inhibition
- PMID: 34478505
- PMCID: PMC8945613
- DOI: 10.1182/bloodadvances.2021005083
Outcomes of patients with CLL sequentially resistant to both BCL2 and BTK inhibition
Abstract
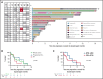
Covalent Bruton tyrosine kinase inhibitors (BTKi's) and the B-cell lymphoma 2 (BCL2) inhibitor venetoclax have significantly improved outcomes for patients with chronic lymphocytic leukemia (CLL), especially those with biologically adverse disease. Patients with CLL resistant to their first targeted agent (TA) can be effectively treated with the alternative class. However, relapses are expected with second-line TA therapy, and the clinical challenge of double class-resistant disease is now emerging with increasing frequency. To define the characteristics and outcomes of patients with double class-resistant disease, we retrospectively analyzed 17 patients who developed progressive disease (PD) on both TA classes for CLL (venetoclax, then BTKi, n=12; BTKi, then venetoclax, n = 5). The cohort was heavily pretreated (median lines of prior therapy, 4) and enriched for adverse disease genetics (complex karyotype, 12 of 12 tested [100%]; del(17p)/TP53 mutations, 15 of 17 [88%]). The median time to progression on prior venetoclax was 24 months (range, 6-94 months) and was 25 months (range, 1-55 months) on prior BTKi. Progression on second-line TA was manifest as progressive CLL in 11 patients and as Richter transformation in 6. The median overall survival after progression on second-line TA was 3.6 months (95% confidence interval, 2-11 months). Patients with double class-resistant CLL have a dismal prognosis, representing a group of high unmet need.
© 2021 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures
References
-
- Moreno C, Greil R, Demirkan F, et al. . Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43-56. - PubMed
-
- Seymour JF, Kipps TJ, Eichhorst B, et al. . Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107-1120. - PubMed
-
- Fischer K, Al-Sawaf O, Bahlo J, et al. . Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225-2236. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

