The aryl hydrocarbon receptor promotes differentiation during mouse preimplantational embryo development
- PMID: 34478649
- PMCID: PMC8452532
- DOI: 10.1016/j.stemcr.2021.08.002
The aryl hydrocarbon receptor promotes differentiation during mouse preimplantational embryo development
Abstract
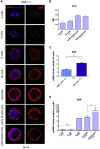
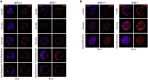
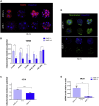
Mammalian embryogenesis is a complex process controlled by transcription factors that regulate the balance between pluripotency and differentiation. Transcription factor aryl hydrocarbon receptor (AhR) regulates OCT4/POU5F1 and NANOG, both essential controllers of pluripotency, stemness and early embryo development. Molecular mechanisms controlling OCT4/POU5F1 and NANOG during embryogenesis remain unidentified. We show that AhR regulates pluripotency factors and maintains the metabolic activity required for proper embryo differentiation. AhR-lacking embryos (AhR-/-) showed a pluripotent phenotype characterized by a delayed expression of trophectoderm differentiation markers. Accordingly, central pluripotency factors OCT4/POU5F1 and NANOG were overexpressed in AhR-/- embryos at initial developmental stages. An altered intracellular localization of these factors was observed in the absence of AhR and, importantly, Oct4 had an opposite expression pattern with respect to AhR from the two-cell stage to blastocyst, suggesting a negative regulation of OCT4/POU5F by AhR. We propose that AhR is a regulator of pluripotency and differentiation in early mouse embryogenesis.
Keywords: Hippo; aryl hydrocarbon receptor; embryo differentiation; pluripotency; preimplantation.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Abbott B.D., Schmid J.E., Pitt J.A., Buckalew A.R., Wood C.R., Held G.A., Diliberto J.J. Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol. Appl. Pharmacol. 1999;155:62–70. - PubMed
-
- Boyer L.A., Mathur D., Jaenisch R. Molecular control of pluripotency. Curr. Opin. Genet. Dev. 2006;16:455–462. - PubMed
-
- Chazaud C., Yamanaka Y. Lineage specification in the mouse preimplantation embryo. Development. 2016;143:1063–1074. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

