Strain differences in the extent of brain injury in mice after tetramethylenedisulfotetramine-induced status epilepticus
- PMID: 34478772
- PMCID: PMC8595842
- DOI: 10.1016/j.neuro.2021.08.011
Strain differences in the extent of brain injury in mice after tetramethylenedisulfotetramine-induced status epilepticus
Abstract
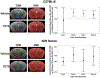
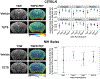
Acute intoxication with tetramethylenedisulfotetramine (TETS) can trigger status epilepticus (SE) in humans. Survivors often exhibit long-term neurological effects, including electrographic abnormalities and cognitive deficits, but the pathogenic mechanisms linking the acute toxic effects of TETS to chronic outcomes are not known. Here, we use advanced in vivo imaging techniques to longitudinally monitor the neuropathological consequences of TETS-induced SE in two different mouse strains. Adult male NIH Swiss and C57BL/6J mice were injected with riluzole (10 mg/kg, i.p.), followed 10 min later by an acute dose of TETS (0.2 mg/kg in NIH Swiss; 0.3 mg/kg, i.p. in C57BL/6J) or an equal volume of vehicle (10% DMSO in 0.9% sterile saline). Different TETS doses were administered to trigger comparable seizure behavior between strains. Seizure behavior began within minutes of TETS exposure and rapidly progressed to SE that was terminated after 40 min by administration of midazolam (1.8 mg/kg, i.m.). The brains of vehicle and TETS-exposed mice were imaged using in vivo magnetic resonance (MR) and translocator protein (TSPO) positron emission tomography (PET) at 1, 3, 7, and 14 days post-exposure to monitor brain injury and neuroinflammation, respectively. When the brain scans of TETS mice were compared to those of vehicle controls, subtle and transient neuropathology was observed in both mouse strains, but more extensive and persistent TETS-induced neuropathology was observed in C57BL/6J mice. In addition, one NIH Swiss TETS mouse that did not respond to the midazolam therapy, but remained in SE for more than 2 h, displayed robust neuropathology as determined by in vivo imaging and confirmed by FluoroJade C staining and IBA-1 immunohistochemistry as readouts of neurodegeneration and neuroinflammation, respectively. These findings demonstrate that the extent of injury observed in the mouse brain after TETS-induced SE varied according to strain, dose of TETS and/or the duration of SE. These observations suggest that TETS-intoxicated humans who do not respond to antiseizure medication are at increased risk for brain injury.
Keywords: MRI; Neurodegeneration; Neuroinflammation; PET; Tetramine.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Baille V, Clarke PG, Brochier G, Dorandeu F, Verna JM, Four E, Lallement G, Carpentier P, 2005. Soman-induced convulsions: the neuropathology revisited. Toxicology. 215, 1–24. - PubMed
-
- Barrueto F Jr., Furdyna PM, Hoffman RS, Hoffman RJ, Nelson LS, 2003. Status epilepticus from an illegally imported Chinese rodenticide: “tetramine”. J. Toxicol. Clin. Toxicol 41, 991–994. - PubMed
-
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300.
-
- Bourdier T, Pham TQ, Henderson D, Jackson T, Lam P, Izard M, Katsifis A, 2012. Automated radiosynthesis of [18F]PBR111 and [18F]PBR102 using the Tracerlab FXFN and Tracerlab MXFDG module for imaging the peripheral benzodiazepine receptor with PET. Appl. Radiat. Isot 70, 176–183. - PubMed

