miRNA induced co-differentiation and cross-talk of adipose tissue-derived progenitor cells for 3D heterotypic pre-vascularized bone formation
- PMID: 34479220
- PMCID: PMC8596330
- DOI: 10.1088/1758-5090/ac23ae
miRNA induced co-differentiation and cross-talk of adipose tissue-derived progenitor cells for 3D heterotypic pre-vascularized bone formation
Abstract
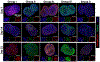
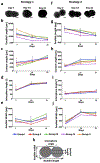
Engineered bone grafts require a vascular network to supply cells with oxygen, nutrients and remove waste. Using heterotypic mature cells to create these graftsin vivohas resulted in limited cell density, ectopic tissue formation and disorganized tissue. Despite evidence that progenitor cell aggregates, such as progenitor spheroids, are a potential candidate for fabrication of native-like pre-vascularized bone tissue, the factors dictating progenitor co-differentiation to create heterotypic pre-vascularized bone tissue remains poorly understood. In this study, we examined a three-dimensional heterotypic pre-vascularized bone tissue model, using osteogenic and endotheliogenic progenitor spheroids induced by miR-148b and miR-210 mimic transfection, respectively. Spheroids made of transfected cells were assembled into heterotypic structures to determine the impact on co-differentiation as a function of micro-RNA (miRNA) mimic treatment group and induction time. Our results demonstrated that miRNAs supported the differentiation in heterotypic structures, and that developing heterotypic structures is determined in part by progenitor maturity, as confirmed by gene and protein markers of osteogenic and endotheliogenic differentiation and the mineralization assay. As a proof of concept, miRNA-transfected spheroids were also bioprinted using aspiration-assisted bioprinting and organized into hollow structures to mimic the Haversian canal. Overall, the presented approach could be useful in fabrication of vascularized bone tissue using spheroids as building blocks.
Keywords: bioprinting; bone; miRNA; pre-vascularization; spheroid assembly; stem cells.
© 2021 IOP Publishing Ltd.
Conflict of interest statement
Conflict of interest
The authors declare no competing interests.
Figures







References
-
- Soucacos PN, Kokkalis ZT, Piagkou M and Johnson EO 2013. Vascularized bone grafts for the management of skeletal defects in orthopaedic trauma and reconstructive surgery Injury 44 S70–5 - PubMed
-
- Cai X, Yang F, Yan X, Yang W, Yu N, Oortgiesen DAW, Wang Y, Jansen JA and Walboomers XF 2015. Influence of bone marrow-derived mesenchymal stem cells pre-implantation differentiation approach on periodontal regeneration in vivo J. Clin. Periodontol 42 380–9 - PubMed
-
- Eppler SM, Combs DL, Henry TD, Lopez JJ, Ellis SG, Yi J-H, Annex BH, McCluskey ER and Zioncheck TF 2002. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans Clin. Pharmacol. Ther 72 20–32 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
