KSHV/HHV8-mediated hematologic diseases
- PMID: 34479367
- PMCID: PMC8854683
- DOI: 10.1182/blood.2020005470
KSHV/HHV8-mediated hematologic diseases
Abstract
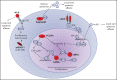
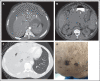
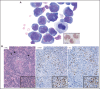
Kaposi sarcoma (KS) herpesvirus (KSHV), also known as human herpesvirus 8, is the causal agent of KS but is also pathogenetically related to several lymphoproliferative disorders, including primary effusion lymphoma (PEL)/extracavitary (EC) PEL, KSHV-associated multicentric Castleman disease (MCD), KSHV+ diffuse large B-cell lymphoma, and germinotropic lymphoproliferative disorder. These different KSHV-associated diseases may co-occur and may have overlapping features. KSHV, similar to Epstein-Barr virus (EBV), is a lymphotropic gammaherpesvirus that is preferentially present in abnormal lymphoid proliferations occurring in immunecompromised individuals. Notably, both KSHV and EBV can infect and transform the same B cell, which is frequently seen in KSHV+ EBV+ PEL/EC-PEL. The mechanisms by which KSHV leads to lymphoproliferative disorders is thought to be related to the expression of a few transforming viral genes that can affect cellular proliferation and survival. There are critical differences between KSHV-MCD and PEL/EC-PEL, the 2 most common KSHV-associated lymphoid proliferations, including viral associations, patterns of viral gene expression, and cellular differentiation stage reflected by the phenotype and genotype of the infected abnormal B cells. Advances in treatment have improved outcomes, but mortality rates remain high. Our deepening understanding of KSHV biology, clinical features of KSHV-associated diseases, and newer clinical interventions should lead to improved and increasingly targeted therapeutic interventions.
© 2022 by The American Society of Hematology.
Figures

References
-
- Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet. 1964;1(7335):702-703. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332(18):1186-1191. - PubMed
-
- Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266(5192):1865-1869. - PubMed
-
- Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86(4):1276-1280. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

