Head and neck squamous cell carcinoma cell lines have an immunomodulatory effect on macrophages independent of hypoxia and toll-like receptor 9
- PMID: 34479492
- PMCID: PMC8418007
- DOI: 10.1186/s12885-021-08357-8
Head and neck squamous cell carcinoma cell lines have an immunomodulatory effect on macrophages independent of hypoxia and toll-like receptor 9
Abstract
Background: A low tissue oxygen level, < 1% O2, is a typical characteristic inside of solid tumors in head and neck cancer (HNSCC) affecting a wide array of cell populations, such as macrophages. However, the mechanisms of how hypoxia influences macrophages are not yet fully elucidated. Our research aimed to study the effect of soluble mediators produced by hypoxic cancer cells on macrophage polarization. Furthermore, we studied the effect of a hypoxic microenvironment on the expression of tumorigenic toll-like receptor 9 (TLR9) and the consecutive macrophage polarization.
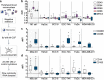
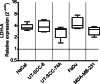
Methods: Conditioned media (CMNOX or CMHOX) from cell lines UT-SCC-8, UT-SCC-74A, FaDu, MDA-MB-231 and HaCat cultured under normoxic (21% O2) and hypoxic (1% O2) conditions were used to polarize human monocyte-derived macrophages. Macrophage polarization was measured by flow cytometry and the production of cytokine mRNA using Taqman qPCR. To study the role of TLR9 in macrophage polarization, the lentiviral CRISPR/Cas9 method was used to establish a stable FaDuTLR9def clone.
Results: Our results demonstrate that the soluble mediators produced by the cancer cells under normoxia polarize macrophages towards a hybridized M1/M2a/M2c phenotype. Furthermore, the results suggest that hypoxia has a limited role in altering the array of cancer-produced soluble factors affecting macrophage polarization and cytokine production. Our data also indicates that increased expression of TLR9 due to hypoxia in malignant cells does not markedly influence the polarization of macrophages. TLR9 transcriptional response to hypoxia is dissimilar to a HIF1-α-regulated LDH-A. This may indicate a context-dependent expression of TLR9 under hypoxia.
Conclusions: HNSCC cell lines affect both macrophage activity (polarization) and functionality (cytokines), but with exception to iNOS expression, the effects appear independent of hypoxia and TLR9.
Keywords: Anti-cancer immunomodulation; Head and neck squamous cell carcinoma; Hypoxia; Immune evasion; Immunoediting; Innate immune response; Macrophage polarization; TLR9.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

