Bovine-associated non-aureus staphylococci suppress Staphylococcus aureus biofilm dispersal in vitro yet not through agr regulation
- PMID: 34479647
- PMCID: PMC8414718
- DOI: 10.1186/s13567-021-00985-z
Bovine-associated non-aureus staphylococci suppress Staphylococcus aureus biofilm dispersal in vitro yet not through agr regulation
Abstract
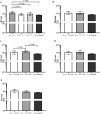
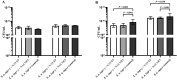
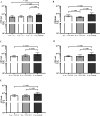
Biofilm formation is a significant virulence factor in Staphylococcus (S.) aureus strains causing subclinical mastitis in dairy cows. A role of environmental signals and communication systems in biofilm development, such as the agr system in S. aureus, is suggested. In the context of multispecies biofilm communities, the presence of non-aureus staphylococci (NAS) might influence S. aureus colonization of the bovine mammary gland, yet, such interspecies interactions have been poorly studied. We determined whether 34 S. chromogenes, 11 S. epidermidis, and 14 S. simulans isolates originating from bovine milk samples and teat apices (TA) were able to affect biofilm formation and dispersion of S. aureus, and if so, how isolate traits such as the capacity to regulate the S. aureus agr quorum sensing system are determinants in this process. The capacity of an agr-positive S. aureus strain to form biofilm was increased more in the presence of S. chromogenes than in the presence of S. simulans and S. epidermidis isolates and in the presence of NAS isolates that do not harbor biofilm related genes. On the other hand, biofilm dispersion of this particular S. aureus strain was suppressed by NAS as a group, an effect that was more pronounced by isolates from TA. Furthermore, the observed effects on biofilm formation and dispersion of the agr-positive S. aureus strain as well as of an agr-negative S. aureus strain did not depend on the capacity of NAS to repress the agr system.
Keywords: Non-aureus staphylococci; Staphylococcus aureus; agr; biofilm; bovine mastitis; quorum sensing.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Hogeveen H, Steeneveld W, Wolf CA. Production diseases reduce the efficiency of dairy production: a review of the results, methods, and approaches regarding the economics of mastitis. Annu Rev Resour Econ. 2019;11:289–312. doi: 10.1146/annurev-resource-100518-093954. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

