Protocol for a multicentre, randomised, parallel-control, superiority trial comparing administration of clotting factor concentrates with a standard massive haemorrhage protocol in severely bleeding trauma patients: the FiiRST 2 trial (a 2020 EAST multicentre trial)
- PMID: 34479938
- PMCID: PMC8420689
- DOI: 10.1136/bmjopen-2021-051003
Protocol for a multicentre, randomised, parallel-control, superiority trial comparing administration of clotting factor concentrates with a standard massive haemorrhage protocol in severely bleeding trauma patients: the FiiRST 2 trial (a 2020 EAST multicentre trial)
Abstract
Introduction: Acute traumatic coagulopathy (ATC) in bleeding trauma patients increase in-hospital mortality. Fibrinogen concentrate (FC) and prothrombin complex concentrate (PCC) are two purified concentrates of clotting factors that have been used to treat ATC. However, there is a knowledge gap on their use compared with the standard of care, the transfusion of plasma.
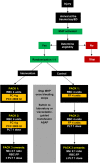
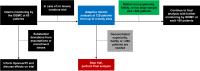
Methods and analysis: The factors in the initial resuscitation of severe trauma 2 trial is a multicentre, randomised, parallel-control, single-blinded, phase IV superiority trial. The study aims to address efficacy and safety of the early use of FC and PCC compared with a plasma-based resuscitation. Adult trauma patients requiring massive haemorrhage protocol activation on hospital arrival will receive FC 4 g and PCC 2000 IU or plasma 4 U, based on random allocation. The primary outcome is a composite of the cumulative number of all units of red cells, plasma and platelets transfused within 24 hours following admission. Secondary outcomes include measures of efficacy and safety of the intervention. Enrolment of 350 patients will provide an initial power >80% to demonstrate superiority for the primary outcome. After enrolment of 120 patients, a preplanned adaptive interim analysis will be conducted to reassess assumptions, check for early superiority demonstration or reassess the sample size for remainder of the study.
Ethics and dissemination: The study has been approved by local and provincial research ethics boards and will be conducted according to the Declaration of Helsinki, Good Clinical Practice guidelines and regulatory requirements. As per the Tri-Council Policy Statement, patient consent will be deferred due to the emergency nature of the interventions. If superiority is established, results will have a major impact on clinical practice by reducing exposure to non-virally inactivated blood products, shortening the time for administration of clotting factors, correct coagulopathy more efficaciously and reduce the reliance on AB plasma.
Trial registration number: NCT04534751, pre results.
Keywords: adult surgery; bleeding disorders & coagulopathies; blood bank & transfusion medicine; protocols & guidelines; trauma management.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LTdL and KK have received support for research and/or honoraria from Octapharma. JCal has received support for research through peer-reviewed grants from Canadian Blood Services. AB is lieutenant colonel in the Royal Canadian Medical Service and receives funding support from CIMVHR. BS is a clinical director at Octapharma.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
