Translational value of choroid plexus imaging for tracking neuroinflammation in mice and humans
- PMID: 34479997
- PMCID: PMC8433504
- DOI: 10.1073/pnas.2025000118
Translational value of choroid plexus imaging for tracking neuroinflammation in mice and humans
Abstract
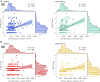
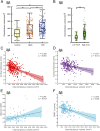
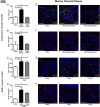
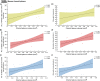
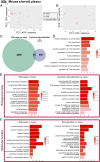
Neuroinflammation is a pathophysiological hallmark of multiple sclerosis and has a close mechanistic link to neurodegeneration. Although this link is potentially targetable, robust translatable models to reliably quantify and track neuroinflammation in both mice and humans are lacking. The choroid plexus (ChP) plays a pivotal role in regulating the trafficking of immune cells from the brain parenchyma into the cerebrospinal fluid (CSF) and has recently attracted attention as a key structure in the initiation of inflammatory brain responses. In a translational framework, we here address the integrity and multidimensional characteristics of the ChP under inflammatory conditions and question whether ChP volumes could act as an interspecies marker of neuroinflammation that closely interrelates with functional impairment. Therefore, we explore ChP characteristics in neuroinflammation in patients with multiple sclerosis and in two experimental mouse models, cuprizone diet-related demyelination and experimental autoimmune encephalomyelitis. We demonstrate that ChP enlargement-reconstructed from MRI-is highly associated with acute disease activity, both in the studied mouse models and in humans. A close dependency of ChP integrity and molecular signatures of neuroinflammation is shown in the performed transcriptomic analyses. Moreover, pharmacological modulation of the blood-CSF barrier with natalizumab prevents an increase of the ChP volume. ChP enlargement is strongly linked to emerging functional impairment as depicted in the mouse models and in multiple sclerosis patients. Our findings identify ChP characteristics as robust and translatable hallmarks of acute and ongoing neuroinflammatory activity in mice and humans that could serve as a promising interspecies marker for translational and reverse-translational approaches.
Keywords: choroid plexus; disease activity; multiple sclerosis; neuroinflammation.
Conflict of interest statement
The authors declare no competing interest.
Figures

Comment in
-
Choroid plexus volumetrics and brain inflammation in multiple sclerosis.Proc Natl Acad Sci U S A. 2021 Oct 5;118(40):e2115221118. doi: 10.1073/pnas.2115221118. Proc Natl Acad Sci U S A. 2021. PMID: 34583997 Free PMC article. No abstract available.
-
A new imaging marker for MS.Lab Anim (NY). 2021 Nov;50(11):308. doi: 10.1038/s41684-021-00880-2. Lab Anim (NY). 2021. PMID: 34697444 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

