Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice
- PMID: 34480023
- PMCID: PMC8417260
- DOI: 10.1038/s41467-021-25453-2
Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice
Abstract
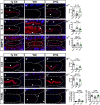
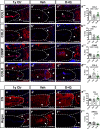
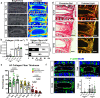
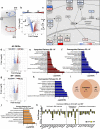
Intervertebral disc degeneration is highly prevalent within the elderly population and is a leading cause of chronic back pain and disability. Due to the link between disc degeneration and senescence, we explored the ability of the Dasatinib and Quercetin drug combination (D + Q) to prevent an age-dependent progression of disc degeneration in mice. We treated C57BL/6 mice beginning at 6, 14, and 18 months of age, and analyzed them at 23 months of age. Interestingly, 6- and 14-month D + Q cohorts show lower incidences of degeneration, and the treatment results in a significant decrease in senescence markers p16INK4a, p19ARF, and SASP molecules IL-6 and MMP13. Treatment also preserves cell viability, phenotype, and matrix content. Although transcriptomic analysis shows disc compartment-specific effects of the treatment, cell death and cytokine response pathways are commonly modulated across tissue types. Results suggest that senolytics may provide an attractive strategy to mitigating age-dependent disc degeneration.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Collaborators GBD. 2017 Disease and Injury Incidence and Prevalence. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:P1789–P1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

