The effect of trichlormethiazide in autosomal dominant polycystic kidney disease patients receiving tolvaptan: a randomized crossover controlled trial
- PMID: 34480075
- PMCID: PMC8417075
- DOI: 10.1038/s41598-021-97113-w
The effect of trichlormethiazide in autosomal dominant polycystic kidney disease patients receiving tolvaptan: a randomized crossover controlled trial
Abstract
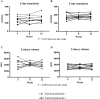
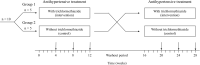
The vasopressin V2 receptor antagonist tolvaptan delays the progression of autosomal dominant polycystic kidney disease (ADPKD). However, some patients discontinue tolvaptan because of severe adverse aquaretic events. This open-label, randomized, controlled, counterbalanced, crossover trial investigated the effects of trichlormethiazide, a thiazide diuretic, in patients with ADPKD receiving tolvaptan (n = 10) who randomly received antihypertensive therapy with or without trichlormethiazide for 12 weeks. The primary and secondary outcomes included amount and osmolarity of 24-h urine and health-related quality-of-life (HRQOL) parameters assessed by the Kidney Disease Quality of Life-Short Form questionnaire, renal function slope, and plasma/urinary biomarkers associated with disease progression. There was a significant reduction in urine volume (3348 ± 584 vs. 4255 ± 739 mL; P < 0.001) and a significant increase in urinary osmolarity (182.5 ± 38.1 vs. 141.5 ± 38.1 mOsm; P = 0.001) in patients treated with trichlormethiazide. Moreover, trichlormethiazide improved the following HRQOL subscales: effects of kidney disease, sleep, emotional role functioning, social functioning, and role/social component summary. No significant differences were noted in renal function slope or plasma/urinary biomarkers between patients treated with and without trichlormethiazide. In patients with ADPKD treated with tolvaptan, trichlormethiazide may improve tolvaptan tolerability and HRQOL parameters.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

