Dendritic cells in cancer immunology
- PMID: 34480145
- PMCID: PMC8752832
- DOI: 10.1038/s41423-021-00741-5
Dendritic cells in cancer immunology
Abstract
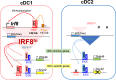
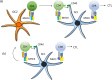
The clinical success of immune checkpoint therapy (ICT) has produced explosive growth in tumor immunology research because ICT was discovered through basic studies of immune regulation. Much of the current translational efforts are aimed at enhancing ICT by identifying therapeutic targets that synergize with CTLA4 or PD1/PD-L1 blockade and are solidly developed on the basis of currently accepted principles. Expanding these principles through continuous basic research may help broaden translational efforts. With this mindset, we focused this review on three threads of basic research directly relating to mechanisms underlying ICT. Specifically, this review covers three aspects of dendritic cell (DC) biology connected with antitumor immune responses but are not specifically oriented toward therapeutic use. First, we review recent advances in the development of the cDC1 subset of DCs, identifying important features distinguishing these cells from other types of DCs. Second, we review the antigen-processing pathway called cross-presentation, which was discovered in the mid-1970s and remains an enigma. This pathway serves an essential in vivo function unique to cDC1s and may be both a physiologic bottleneck and therapeutic target. Finally, we review the longstanding field of helper cells and the related area of DC licensing, in which CD4 T cells influence the strength or quality of CD8 T cell responses. Each topic is connected with ICT in some manner but is also a fundamental aspect of cell-mediated immunity directed toward intracellular pathogens.
Keywords: CD4 help; Dendritic cells; cross-presentation; transcription factors; tumor rejection.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ardavin C, Shortman K. Cell surface marker analysis of mouse thymic dendritic cells. Eur J Immunol. 1992;22:859–62. - PubMed
-
- Crozat K, Tamoutounour S, Vu Manh TP, Fossum E, Luche H, Ardouin L, et al. Cutting edge: expression of XCR1 defines mouse lymphoid-tissue resident and migratory dendritic cells of the CD8alpha+ type. J Immunol. 2011;187:4411–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

