Preoperative systemic chemotherapy alters the histopathological growth patterns of colorectal liver metastases
- PMID: 34480530
- PMCID: PMC8682940
- DOI: 10.1002/cjp2.235
Preoperative systemic chemotherapy alters the histopathological growth patterns of colorectal liver metastases
Abstract
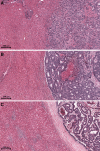
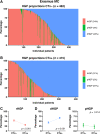
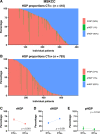
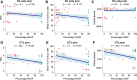
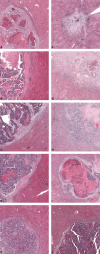
Histopathological growth patterns (HGPs) are a reliable, reproducible, and strong prognostic biomarker that can be assessed on haematoxylin and eosin-stained sections of resected colorectal liver metastases (CRLM). Assessment estimates the relative fraction of the tumour-liver interface for each of the three growth patterns; the desmoplastic HGP reflects good prognosis. Whether preoperative chemotherapy affects the HGP is currently unclear. The present international multicentre study evaluates this in an original cohort of 877 consecutive patients treated in the Netherlands, an external validation cohort of 1,203 consecutive patients treated in the USA, and a post hoc analysis from the phase III randomised controlled European Organization for Research and Treatment of Cancer (EORTC) 40983 trial (n = 70). All patients underwent resection of CRLM with or without preoperative systemic chemotherapy. Trial patients were randomised between perioperative chemotherapy and resection or resection alone. HGPs were determined according to consensus guidelines and compared for preoperative treatment status. Data from three separate tumour regression grading systems were available for the trial cohort. These were correlated with HGP stratified for treatment arm. In the original cohort, the average presence of desmoplastic HGP was 43% for chemo-naïve versus 67% for preoperatively treated patients (p < 0.001). A significant association between chemotherapy and desmoplastic HGP was found on multivariable analysis (β [95% confidence interval, CI]: 24.57 [18.28-30.87], p < 0.001). In the validation cohort, the average presence of desmoplastic HGP was 40% for chemo-naïve versus 63% for preoperatively treated patients (p < 0.001). This association remained on multivariable analysis (β [95% CI]: 24.18 [18.70-29.66], p < 0.001). In the EORTC 40983 trial, the average desmoplastic HGP presence was 33% in the resection arm versus 61% in the chemotherapy arm (p = 0.005). Chemotherapy was independently associated with an increase in desmoplastic HGP (β [95% CI]: 23.29 [1.78-44.79], p = 0.022). All three tumour regression gradings were significantly associated with the desmoplastic HGP in the chemotherapy arm (all p < 0.04). None were associated in the resection arm (all p > 0.11). Preoperative chemotherapy induces histopathological changes that alter the HGP of CRLM.
Keywords: colorectal cancer; colorectal liver metastases; histopathological growth patterns; systemic chemotherapy.
© 2021 The Authors. The Journal of Pathology: Clinical Research published by The Pathological Society of Great Britain and Ireland & John Wiley & Sons, Ltd.
Figures

References
-
- Brunner SM, Kesselring R, Rubner C, et al. Prognosis according to histochemical analysis of liver metastases removed at liver resection. Br J Surg 2014; 101: 1681–1691. - PubMed
-
- Eefsen RL, Vermeulen PB, Christensen IJ, et al. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin Exp Metastasis 2015; 32: 369–381. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

