Effectiveness, Safety, and Tolerability of Nabiximols Oromucosal Spray vs Typical Oral Long-Acting Opioid Analgesics in Patients with Severe Neuropathic Back Pain: Analysis of 6-Month Real-World Data from the German Pain e-Registry
- PMID: 34480564
- PMCID: PMC8992580
- DOI: 10.1093/pm/pnab263
Effectiveness, Safety, and Tolerability of Nabiximols Oromucosal Spray vs Typical Oral Long-Acting Opioid Analgesics in Patients with Severe Neuropathic Back Pain: Analysis of 6-Month Real-World Data from the German Pain e-Registry
Abstract
Objective: To compare the effectiveness, safety, and tolerability of add-on nabiximols (NBX) oromucosal spray vs typical oral long-acting opioid (LAO) analgesics in patients with severe (± chronic) peripheral neuropathic back pain poorly responsive to other treatments.
Methods: Retrospective analysis of anonymized, propensity score-matched data from the German Pain e-Registry of adult outpatients who initiated NBX or LAO between March 2017 and March 2020.
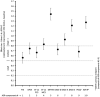
Results: Data were analyzed from propensity score-matched patients treated with NBX (n = 655) or LAO (n = 655): mean age ≈51 years; 57% female; mean pain duration ≈2.6 years; chronic pain 61%; severe dysfunctional pain 93%. At 6 months, NBX was noninferior to LAO for overall symptom relief, based on the least-squares mean difference between cohorts in change from baseline in patient-reported, pain-related aggregated nine-item scale scores (-27.84%; 95% confidence interval [CI] -29.71 to -25.96; P < 0.001) and individual pain-related scale scores. Subsequent prespecified superiority analysis of the primary endpoint showed that NBX was superior to LAO: all secondary endpoints measuring symptoms of pain and physical function improved significantly with NBX and LAO, with between-group differences favoring NBX (all P < 0.001). Fewer patients treated with NBX than LAO experienced treatment-related adverse events (25.5% vs 76.0%; P < 0.001) or discontinued treatment because of treatment-related adverse events (7.9% vs 29.3%; P < 0.001).
Conclusion: Within study limitations (e.g., observational design, all potential biases), add-on NBX was superior to and better tolerated than add-on treatment with typical oral LAO analgesics in patients with neuropathic back pain inadequately controlled by recommended/established systemic therapies.
Keywords: Long-Acting Opioid Analgesics; Low Back Pain; Nabiximols; Neuropathic Pain; Oromucosal Spray; Real-World Data.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Academy of Pain Medicine.
Figures



References
-
- Freynhagen R, Baron R, Gockel U, Tölle TR.. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22(10):1911–20. - PubMed
-
- Freynhagen R, Baron R, Tölle T, et al.Screening of neuropathic pain components in patients with chronic back pain associated with nerve root compression: A prospective observational pilot study (MIPORT). Curr Med Res Opin 2006;22(3):529–37. - PubMed
-
- Torrance N, Smith BH, Bennett MI, Lee AJ.. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain 2006;7(4):281–9. - PubMed

