Persistence of the Immune Responses and Cross-Neutralizing Activity With Variants of Concern Following 2 Doses of Adjuvanted SCB-2019 Coronavirus Disease 2019 Vaccine
- PMID: 34480575
- PMCID: PMC8499965
- DOI: 10.1093/infdis/jiab447
Persistence of the Immune Responses and Cross-Neutralizing Activity With Variants of Concern Following 2 Doses of Adjuvanted SCB-2019 Coronavirus Disease 2019 Vaccine
Abstract
Background: We have previously reported the safety and immunogenicity 4 weeks after 2 doses of the Clover coronavirus disease 2019 (COVID-19) vaccine candidate, SCB-2019, a stabilized prefusion form of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein (S-trimer). We now report persistence of antibodies up to 6 months after vaccination, and cross-neutralization titers against 3 variants of concern (VoCs).
Methods: In a phase 1 study, adult (18-54 years of age) and elderly (55-75 years of age) volunteers received 2 vaccinations 21 days apart with placebo or 3-, 9-, or 30-µg. We measured immunoglobulin G (IgG) antibodies against SCB-2019, angiotensin-converting enzyme 2 (ACE2) competitive binding antibodies, and neutralizing antibodies against wild-type SARS-CoV-2 (Wuhan-Hu-1) at days 101 and 184, and neutralizing antibodies against 3 VoCs, Alpha (B.1.1.7), Beta (B.1.351), and Gamma (P.1), in day 36 sera.
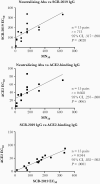
Results: Titers waned from their peak at days 36-50, but SCB-2019 IgG antibodies, ACE2 competitive binding antibodies, and neutralizing antibodies against wild-type SARS-CoV-2 persisted at 25%-35% of their observed peak levels at day 184. Day 36 sera also demonstrated dose-dependent increases in neutralizing titers against the 3 VoCs.
Conclusions: SCB-2019 dose-dependently induced immune responses against wild-type SARS-CoV-2, which persisted up to day 184. Neutralizing antibodies were cross-reactive against 3 of the most prevalent VoCs.
Keywords: COVID-19; immunogenicity; persistence; vaccine; variants of concern.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


References
-
- Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England; technical briefing 15, 11 June 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploa.... Accessed 16 September 2021.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

