Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002)
- PMID: 34480858
- PMCID: PMC8409975
- DOI: 10.1016/S0140-6736(21)01699-8
Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002)
Abstract
Background: COVID-19 vaccine supply shortages are causing concerns about compromised immunity in some countries as the interval between the first and second dose becomes longer. Conversely, countries with no supply constraints are considering administering a third dose. We assessed the persistence of immunogenicity after a single dose of ChAdOx1 nCoV-19 (AZD1222), immunity after an extended interval (44-45 weeks) between the first and second dose, and response to a third dose as a booster given 28-38 weeks after the second dose.
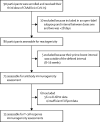
Methods: In this substudy, volunteers aged 18-55 years who were enrolled in the phase 1/2 (COV001) controlled trial in the UK and had received either a single dose or two doses of 5 × 1010 viral particles were invited back for vaccination. Here we report the reactogenicity and immunogenicity of a delayed second dose (44-45 weeks after first dose) or a third dose of the vaccine (28-38 weeks after second dose). Data from volunteers aged 18-55 years who were enrolled in either the phase 1/2 (COV001) or phase 2/3 (COV002), single-blinded, randomised controlled trials of ChAdOx1 nCoV-19 and who had previously received a single dose or two doses of 5 × 1010 viral particles are used for comparison purposes. COV001 is registered with ClinicalTrials.gov, NCT04324606, and ISRCTN, 15281137, and COV002 is registered with ClinicalTrials.gov, NCT04400838, and ISRCTN, 15281137, and both are continuing but not recruiting.
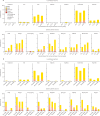
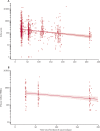
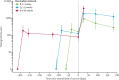
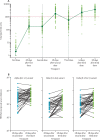
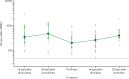
Findings: Between March 11 and 21, 2021, 90 participants were enrolled in the third-dose boost substudy, of whom 80 (89%) were assessable for reactogenicity, 75 (83%) were assessable for evaluation of antibodies, and 15 (17%) were assessable for T-cells responses. The two-dose cohort comprised 321 participants who had reactogenicity data (with prime-boost interval of 8-12 weeks: 267 [83%] of 321; 15-25 weeks: 24 [7%]; or 44-45 weeks: 30 [9%]) and 261 who had immunogenicity data (interval of 8-12 weeks: 115 [44%] of 261; 15-25 weeks: 116 [44%]; and 44-45 weeks: 30 [11%]). 480 participants from the single-dose cohort were assessable for immunogenicity up to 44-45 weeks after vaccination. Antibody titres after a single dose measured approximately 320 days after vaccination remained higher than the titres measured at baseline (geometric mean titre of 66·00 ELISA units [EUs; 95% CI 47·83-91·08] vs 1·75 EUs [1·60-1·93]). 32 participants received a late second dose of vaccine 44-45 weeks after the first dose, of whom 30 were included in immunogenicity and reactogenicity analyses. Antibody titres were higher 28 days after vaccination in those with a longer interval between first and second dose than for those with a short interval (median total IgG titre: 923 EUs [IQR 525-1764] with an 8-12 week interval; 1860 EUs [917-4934] with a 15-25 week interval; and 3738 EUs [1824-6625] with a 44-45 week interval). Among participants who received a third dose of vaccine, antibody titres (measured in 73 [81%] participants for whom samples were available) were significantly higher 28 days after a third dose (median total IgG titre: 3746 EUs [IQR 2047-6420]) than 28 days after a second dose (median 1792 EUs [IQR 899-4634]; Wilcoxon signed rank test p=0·0043). T-cell responses were also boosted after a third dose (median response increased from 200 spot forming units [SFUs] per million peripheral blood mononuclear cells [PBMCs; IQR 127-389] immediately before the third dose to 399 SFUs per milion PBMCs [314-662] by day 28 after the third dose; Wilcoxon signed rank test p=0·012). Reactogenicity after a late second dose or a third dose was lower than reactogenicity after a first dose.
Interpretation: An extended interval before the second dose of ChAdOx1 nCoV-19 leads to increased antibody titres. A third dose of ChAdOx1 nCoV-19 induces antibodies to a level that correlates with high efficacy after second dose and boosts T-cell responses.
Funding: UK Research and Innovation, Engineering and Physical Sciences Research Council, National Institute for Health Research, Coalition for Epidemic Preparedness Innovations, National Institute for Health Research Oxford Biomedical Research Centre, Chinese Academy of Medical Sciences Innovation Fund for Medical Science, Thames Valley and South Midlands NIHR Clinical Research Network, AstraZeneca, and Wellcome.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests SCG and AVSH are cofounders of and shareholders in Vaccitech (collaborators in the early development of ChAdOx1 nCoV-19) and named as inventors on a patent covering use of ChAdOx1-vectored vaccines (PCT/GB2012/000467) and a patent application covering this SARS-CoV-2 vaccine (SCG only). TL is named as an inventor on a patent covering use of ChAdOx1-vectored vaccines (PCT/GB2012/000467) and was a consultant to Vaccitech. PMF is a consultant to Vaccitech. AJP is chair of the UK Department of Health and Social Care's Joint Committee on Vaccination and Immunisation, but does not participate in policy advice on coronavirus vaccines, and is a member of the WHO Strategic Advisory Group of Experts (SAGE). AJP is an NIHR Senior Investigator. All other authors declare no competing interests.
Figures
Comment in
-
Longer intervals and extra doses of ChAdOx1 nCoV-19 vaccine.Lancet. 2021 Sep 11;398(10304):933-935. doi: 10.1016/S0140-6736(21)01817-1. Epub 2021 Sep 1. Lancet. 2021. PMID: 34480860 Free PMC article. No abstract available.
References
-
- WHO . World Health Organization; Geneva: Feb 10, 2021. Interim recommendations for use of the AZD1222 (ChAdOx1-S [recombinant]) vaccine against COVID19 developed by Oxford University and AstraZeneca.https://apps.who.int/iris/bitstream/handle/10665/339477/WHO-2019-nCoV-va...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

