Spreading of Alzheimer tau seeds is enhanced by aging and template matching with limited impact of amyloid-β
- PMID: 34480901
- PMCID: PMC8477193
- DOI: 10.1016/j.jbc.2021.101159
Spreading of Alzheimer tau seeds is enhanced by aging and template matching with limited impact of amyloid-β
Abstract
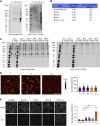
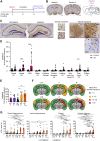
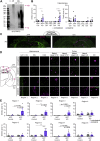
In Alzheimer's disease (AD), deposition of pathological tau and amyloid-β (Aβ) drive synaptic loss and cognitive decline. The injection of misfolded tau aggregates extracted from human AD brains drives templated spreading of tau pathology within WT mouse brain. Here, we assessed the impact of Aβ copathology, of deleting loci known to modify AD risk (Ptk2b, Grn, and Tmem106b) and of pharmacological intervention with an Fyn kinase inhibitor on tau spreading after injection of AD tau extracts. The density and spreading of tau inclusions triggered by human tau seed were unaltered in the hippocampus and cortex of APPswe/PSEN1ΔE9 transgenic and AppNL-F/NL-F knock-in mice. In mice with human tau sequence replacing mouse tau, template matching enhanced neuritic tau burden. Human AD brain tau-enriched preparations contained aggregated Aβ, and the Aβ coinjection caused a redistribution of Aβ aggregates in mutant AD model mice. The injection-induced Aβ phenotype was spatially distinct from tau accumulation and could be ameliorated by depleting Aβ from tau extracts. These data suggest that Aβ and tau pathologies propagate by largely independent mechanisms after their initial formation. Altering the activity of the Fyn and Pyk2 (Ptk2b) kinases involved in Aβ-oligomer-induced signaling, or deleting expression of the progranulin and TMEM106B lysosomal proteins, did not alter the somatic tau inclusion burden or spreading. However, mouse aging had a prominent effect to increase the accumulation of neuritic tau after injection of human AD tau seeds into WT mice. These studies refine our knowledge of factors capable of modulating tau spreading.
Keywords: Alzheimer's disease; amyloid-beta; stereotactic injection; tau protein; transgenic mice.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest S. M. S. is an inventor on a patent application related to the use of Fyn kinase inhibitors in AD and is a cofounder and holds equity interest in Allyx Therapeutics, seeking to develop Alzheimer's therapies. The other authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. - PubMed
-
- Alzheimer's Association 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327–406. - PubMed
-
- Lee V.M., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. - PubMed
-
- Kurt M.A., Davies D.C., Kidd M. Paired helical filament morphology varies with intracellular location in Alzheimer's disease brain. Neurosci. Lett. 1997;239:41–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

