G protein-coupled receptor-effector macromolecular membrane assemblies (GEMMAs)
- PMID: 34480967
- PMCID: PMC9375844
- DOI: 10.1016/j.pharmthera.2021.107977
G protein-coupled receptor-effector macromolecular membrane assemblies (GEMMAs)
Abstract
G protein-coupled receptors (GPCRs) are the largest group of receptors involved in cellular signaling across the plasma membrane and a major class of drug targets. The canonical model for GPCR signaling involves three components - the GPCR, a heterotrimeric G protein and a proximal plasma membrane effector - that have been generally thought to be freely mobile molecules able to interact by 'collision coupling'. Here, we synthesize evidence that supports the existence of GPCR-effector macromolecular membrane assemblies (GEMMAs) comprised of specific GPCRs, G proteins, plasma membrane effector molecules and other associated transmembrane proteins that are pre-assembled prior to receptor activation by agonists, which then leads to subsequent rearrangement of the GEMMA components. The GEMMA concept offers an alternative and complementary model to the canonical collision-coupling model, allowing more efficient interactions between specific signaling components, as well as the integration of the concept of GPCR oligomerization as well as GPCR interactions with orphan receptors, truncated GPCRs and other membrane-localized GPCR-associated proteins. Collision-coupling and pre-assembled mechanisms are not exclusive and likely both operate in the cell, providing a spectrum of signaling modalities which explains the differential properties of a multitude of GPCRs in their different cellular environments. Here, we explore the unique pharmacological characteristics of individual GEMMAs, which could provide new opportunities to therapeutically modulate GPCR signaling.
Keywords: G protein subnits; G protein-coupled receptors; GPCR allosterism; GPCR oligomerization; Plasma membrane effector.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest J.G.-M. has a sponsored research contract with Neuristic Pharma. The remaining authors declare that there are no conflicts of interest.
Figures



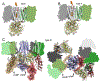
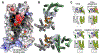


References
-
- Ahmed SN, Brown DA, & London E (1997). On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry, 36(36), 10944–10953. - PubMed
-
- Andressen KW, Ulsund AH, Krobert KA, Lohse MJ, Bünemann M, & Levy FO (2018). Related GPCRs couple differently to Gs: preassociation between G protein and 5-HT7 serotonin receptor reveals movement of Gαs upon receptor activation. FASEB Journal, 32(2), 1059–1069. - PubMed
-
- Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, et al. (2002). Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. The Journal of Biological Chemistry, 277(24), 21522–21528. - PubMed
-
- Ayoub MA, Levoye A, Delagrange P, & Jockers R (2004). Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Molecular Pharmacology, 66(2), 312–321. - PubMed
-
- Ayoub MA, Trinquet E, Pfleger KD, & Pin JP (2010). Differential association modes of the thrombin receptor PAR1 with Galphai1, Galpha12, and beta-arrestin 1. FASEB Journal, 24(9), 3522–3535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

