Toward optoacoustic sciatic nerve detection using an all-fiber interferometric-based sensor for endoscopic smart laser surgery
- PMID: 34481417
- PMCID: PMC9293106
- DOI: 10.1002/lsm.23473
Toward optoacoustic sciatic nerve detection using an all-fiber interferometric-based sensor for endoscopic smart laser surgery
Abstract
Objectives: Laser surgery requires efficient tissue classification to reduce the probability of undesirable or unwanted tissue damage. This study aimed to investigate acoustic shock waves (ASWs) as a means of classifying sciatic nerve tissue.
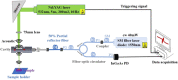
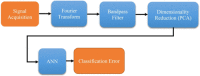
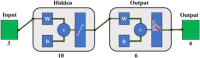
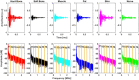
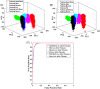
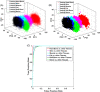
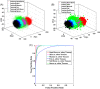
Materials and methods: In this study, we classified sciatic nerve tissue against other tissue types-hard bone, soft bone, fat, muscle, and skin extracted from two proximal and distal fresh porcine femurs-using the ASWs generated by a laser during ablation. A nanosecond frequency-doubled Nd:YAG laser at 532 nm was used to create 10 craters on each tissue type's surface. We used a fiber-coupled Fabry-Pérot sensor to measure the ASWs. The spectrum's amplitude from each ASW frequency band measured was used as input for principal component analysis (PCA). PCA was combined with an artificial neural network to classify the tissue types. A confusion matrix and receiver operating characteristic (ROC) analysis was used to calculate the accuracy of the testing-data-based scores from the sciatic nerve and the area under the ROC curve (AUC) with a 95% confidence-level interval.
Results: Based on the confusion matrix and ROC analysis of the model's tissue classification results (leave-one-out cross-validation), nerve tissue could be classified with an average accuracy rate and AUC result of 95.78 ± 1.3% and 99.58 ± 0.6%, respectively.
Conclusion: This study demonstrates the potential of using ASWs for remote classification of nerve and other tissue types. The technique can serve as the basis of a feedback control system to detect and preserve sciatic nerves in endoscopic laser surgery.
Keywords: acoustic shock signal; artificial network machine; laser ablation; principal component analysis; sciatic nerve tissue; tissue classification.
© 2021 The Authors. Lasers in Surgery and Medicine published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures




References
-
- Kuttenberger JJ, Stübinger S, Waibel A, Werner M, Klasing M, Ivanenko M, et al. Computer‐guided CO2‐laser osteotomy of the sheep tibia: technical prerequisites and first results. Photomed Laser Surg. 2008;26(2):129–36. - PubMed
-
- Kenhagho HN, Canbaz F, Guzman R, Cattin P, Zam A. Miniaturized optoacoustic feedback sensor for smart laser osteotome: fiber‐coupled Fabry‐Pérot etalon sensor. Sens Actuators, A. 2020;317:112394. 10.1016/j.sna.2020.112394 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

